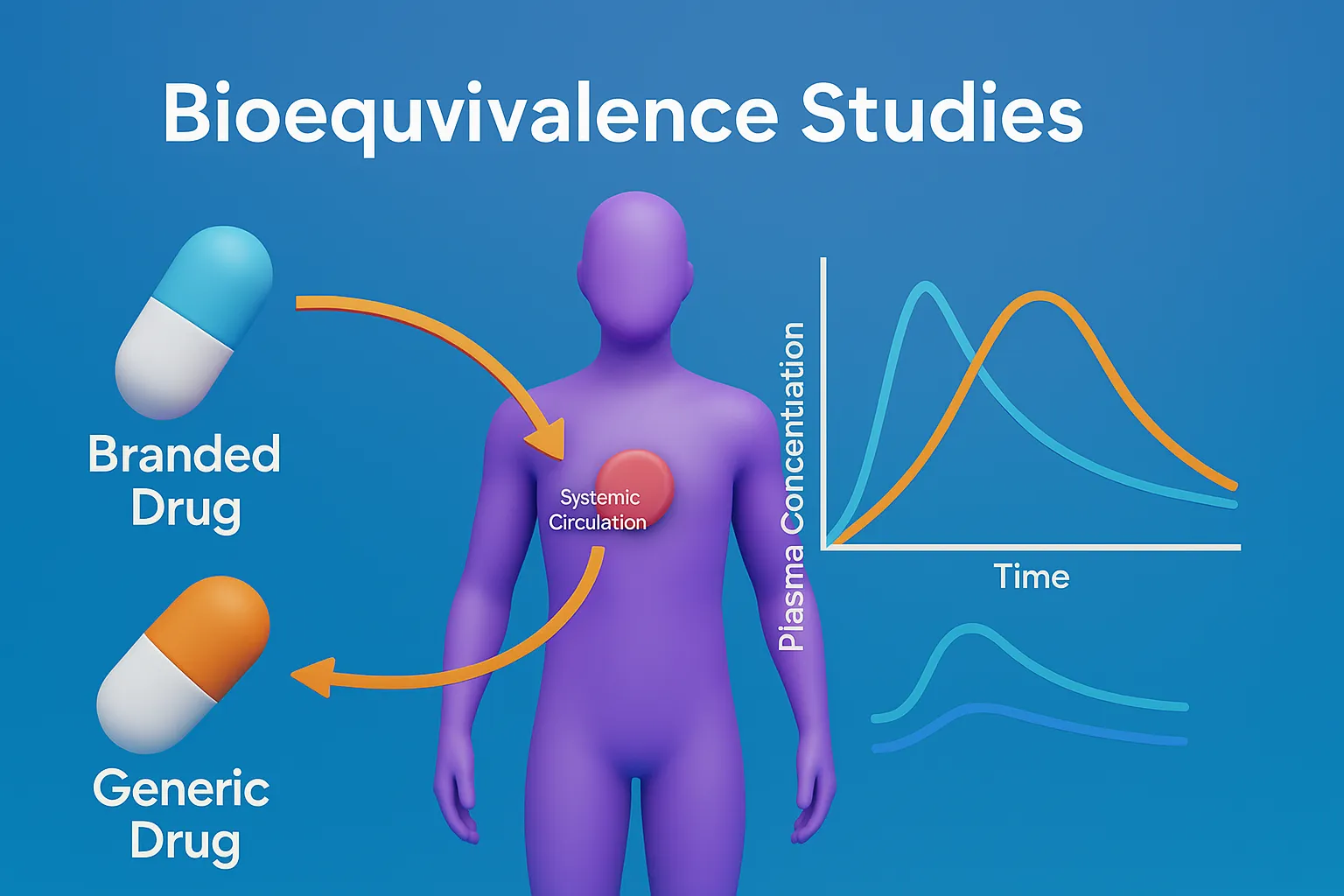
Bioequivalence Studies compare drug products to ensure similar bioavailability, therapeutic effect, and safety in patients.
Bioequivalence (BE)
- Bioequivalence ensures that two drug formulations have similar pharmacokinetic properties, leading to the same safety and efficacy when administered under identical conditions.
- It is a subset of therapeutic equivalence, focusing on rate and extent of drug absorption.
Types of Equivalence
-
- Pharmaceutical Equivalence – Same active ingredient, dosage form, strength, and route of administration.
- Therapeutic Equivalence – Pharmaceutical equivalents with comparable safety and efficacy.
- Bioequivalence – Pharmaceutical equivalents with similar pharmacokinetic profiles (AUC, Cmax, Tmax).
Bioequivalence Studies
- Bioequivalence studies compare a test drug (generic) to a reference drug (brand-name) to confirm similar in-vivo performance and ensure the generic provides the same therapeutic effect.
Objective of BE Studies
-
- Demonstrate comparable pharmacokinetics between test and reference products.
- Compare AUC (Area Under the Curve), Cmax (Maximum Concentration), and Tmax (Time to Cmax) to assess absorption similarity.
Types of BE Studies
- In-Vitro Studies – Dissolution testing under lab conditions to predict in-vivo behavior.
- In-Vivo Studies – Compare AUC, Cmax, Tmax in humans or animals to assess drug absorption and bioavailability.
Methods of Bioequivalence Testing
- Pharmacokinetic Studies – Measure AUC, Cmax, Tmax in healthy volunteers.
- Pharmacodynamic Studies – Compare drug effects (used for topical or inhaled drugs).
- Clinical Trials – Rarely used, required for drugs with complex effects.
- In-Vitro Dissolution Testing – Used when IVIVC is established.
Regulatory Criteria (FDA, EMA)
- AUC & Cmax must fall within 80-125
- Ensures therapeutic equivalence between generic and brand-name drugs.
Click Here to Watch the Best Pharma Videos