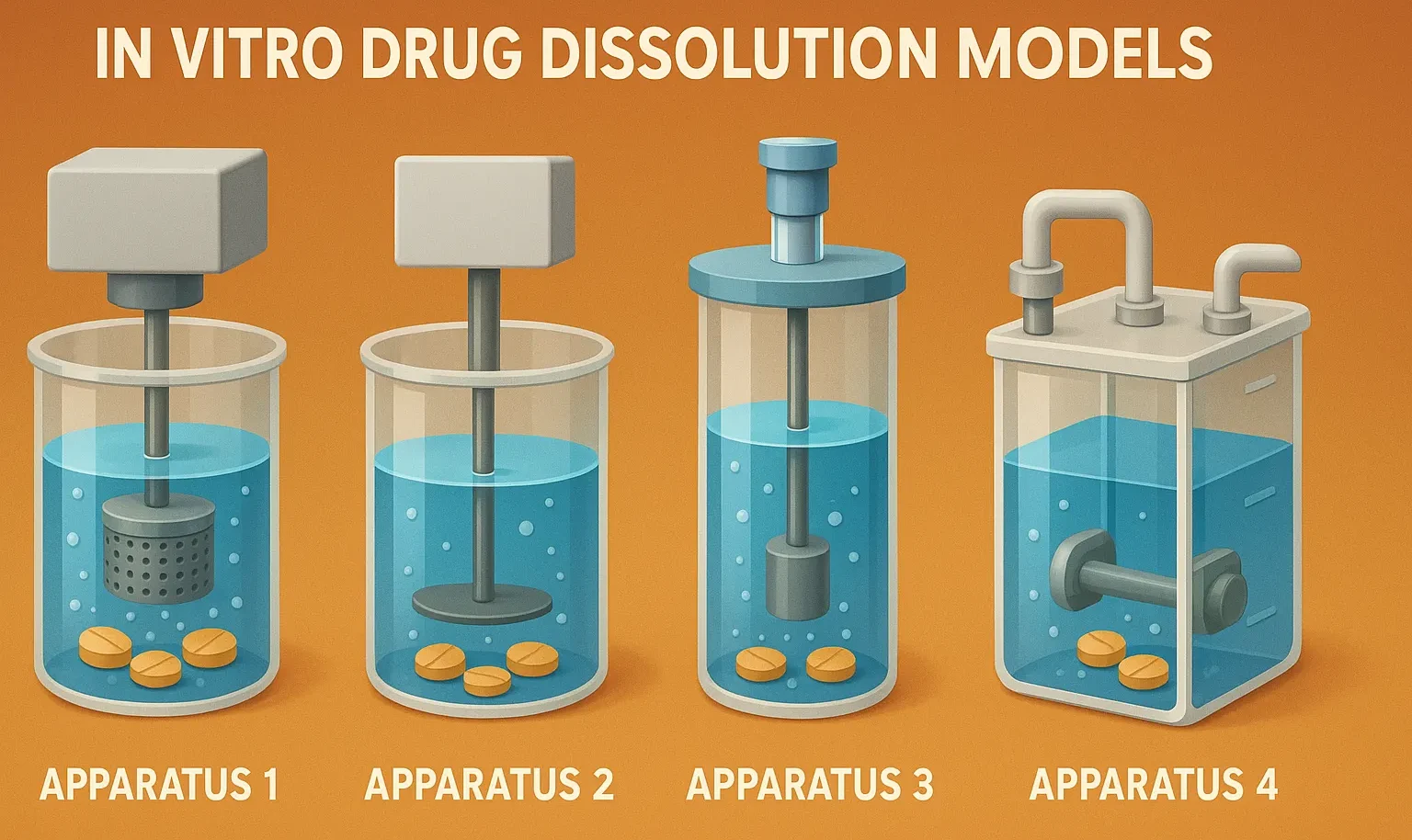
In vitro drug dissolution models evaluate drug release from dosage forms using methods like USP apparatus for bioavailability prediction.
In-vitro drug dissolution models
- In-vitro drug dissolution models are essential in pharmaceutical research to study drug release from formulations like tablets and capsules.
- These models help predict drug dissolution, absorption, and bioavailability, ensuring consistent drug quality.
Principle of a Dissolution Apparatus
- Simulates gastrointestinal conditions to study drug release.
- Maintains controlled temperature and agitation for dissolution.
- Measures drug concentration over time to assess release, absorption, and bioavailability.
Ideal Apparatus Features
- Maintains 37°C, ensures consistent agitation, allows easy sampling, supports varied dosage forms, and meets regulatory standards.
Advertisements
Types of Dissolution Apparatus
- USP 1 (Basket) – Rotating basket in a dissolution medium.
- USP 2 (Paddle) – Uses paddle stirring for uniform mixing.
- USP 3 (Reciprocating Cylinder) – Mimics GI motility, ideal for modified-release drugs.
- USP 4 (Flow-Through Cell) – Continuous medium flow, good for poorly soluble drugs.
- Rotating Disk/Cylinder – Studies surface area impact on dissolution.
- GI Simulator – Advanced model replicating stomach, intestines, pH variations, enzymes for realistic absorption predictions.
Factors Affecting Performance
- Apparatus Type – Choice depends on drug and dosage form (e.g., USP 1, 2, Flow-Through Cell).
- Dissolution Medium – pH, composition, and volume should mimic physiological fluids.
- Temperature – Maintained at 37°C for accuracy.
- Agitation Rate – Controlled basket/paddle speed or flow rate.
- Sampling & Analysis – HPLC, UV spectrophotometry ensure precise drug concentration measurement