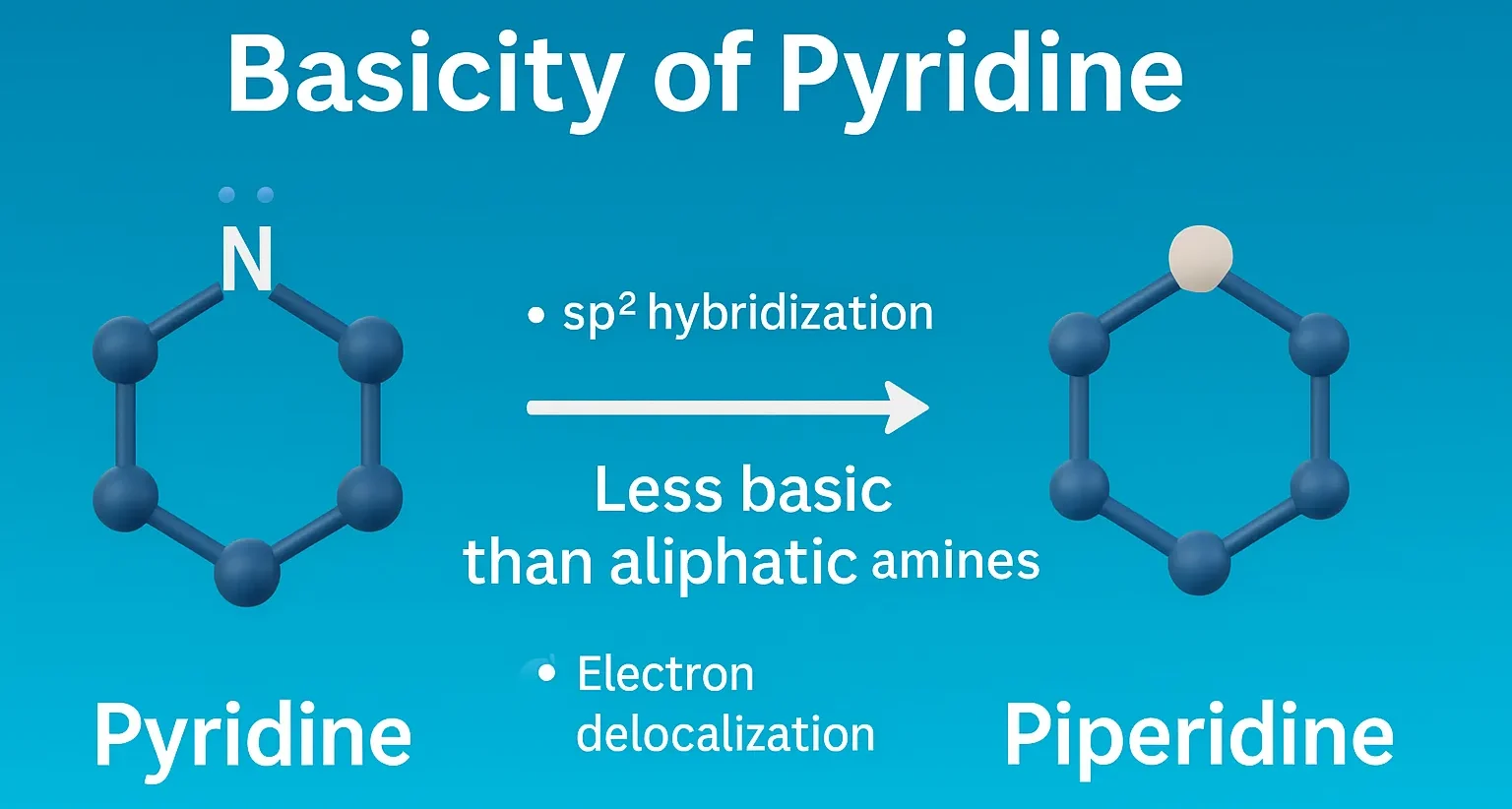
- Basicity of Pyridine explains its nitrogen lone pair, resonance effects, and comparison with pyrrole and aliphatic amines.
- The basicity of pyridine refers to its ability to accept a proton (H⁺), which depends on the availability of its lone pair of electrons on the nitrogen atom.
Structure and Basicity
Pyridine is an aromatic heterocycle with the formula C₅H₅N. It’s similar to benzene, but one CH group is replaced by a nitrogen atom.
Advertisements
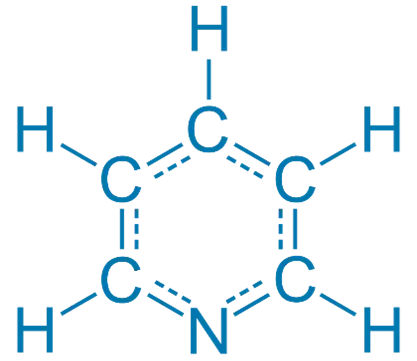
Here’s how its structure influences its basicity:
Advertisements
-
Lone Pair on Nitrogen:
- The nitrogen in pyridine is sp² hybridized.
- Its lone pair of electrons is in an sp² orbital perpendicular to the π system of the aromatic ring.
- This lone pair does not participate in aromaticity, so it is available to accept protons (H⁺) — making pyridine a Lewis base.
-
Basicity Compared to Other Compounds:
- Pyridine is less basic than aliphatic amines (like methylamine) because the nitrogen is more electronegative due to its inclusion in an aromatic ring.
- It is more basic than pyrrole, where the nitrogen lone pair is part of the aromatic sextet and thus not available for protonation.
-
pKa of the Conjugate Acid:
- The pKa of the conjugate acid of pyridine (pyridinium ion) is about 2, indicating it is a weak base.
Click Here to Watch the Best Pharma Videos
Advertisements