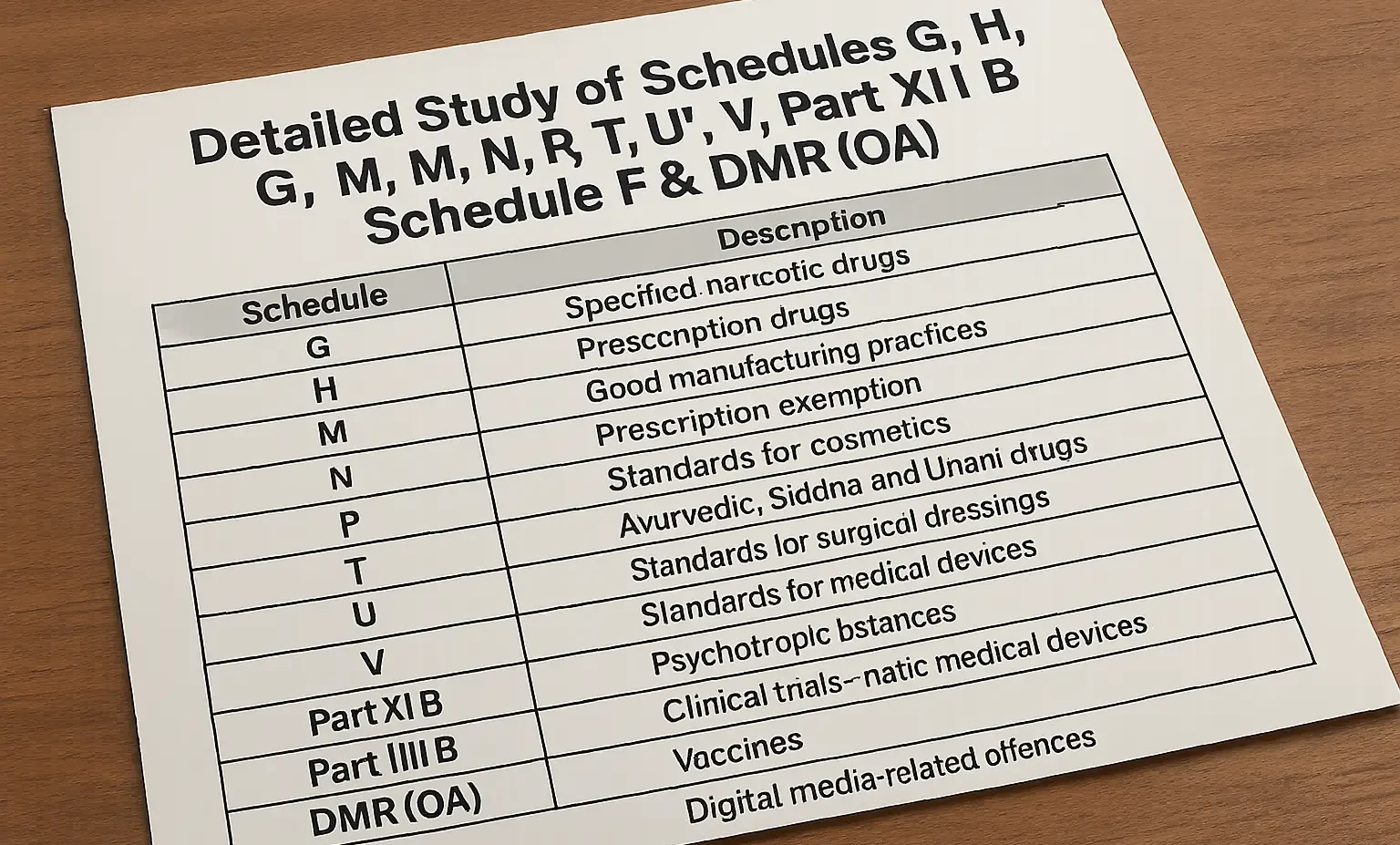
General Labeling Requirements
Understand the general labeling requirements for drugs and cosmetics under Indian law to ensure legal compliance and consumer safety. General Labeling Requirements Proper labeling is crucial for ensuring that consumers have accurate and essential information about the drugs and cosmetics they use. The Drugs and Cosmetics Act, 1940 lays out comprehensive labeling standards to safeguard … Read more