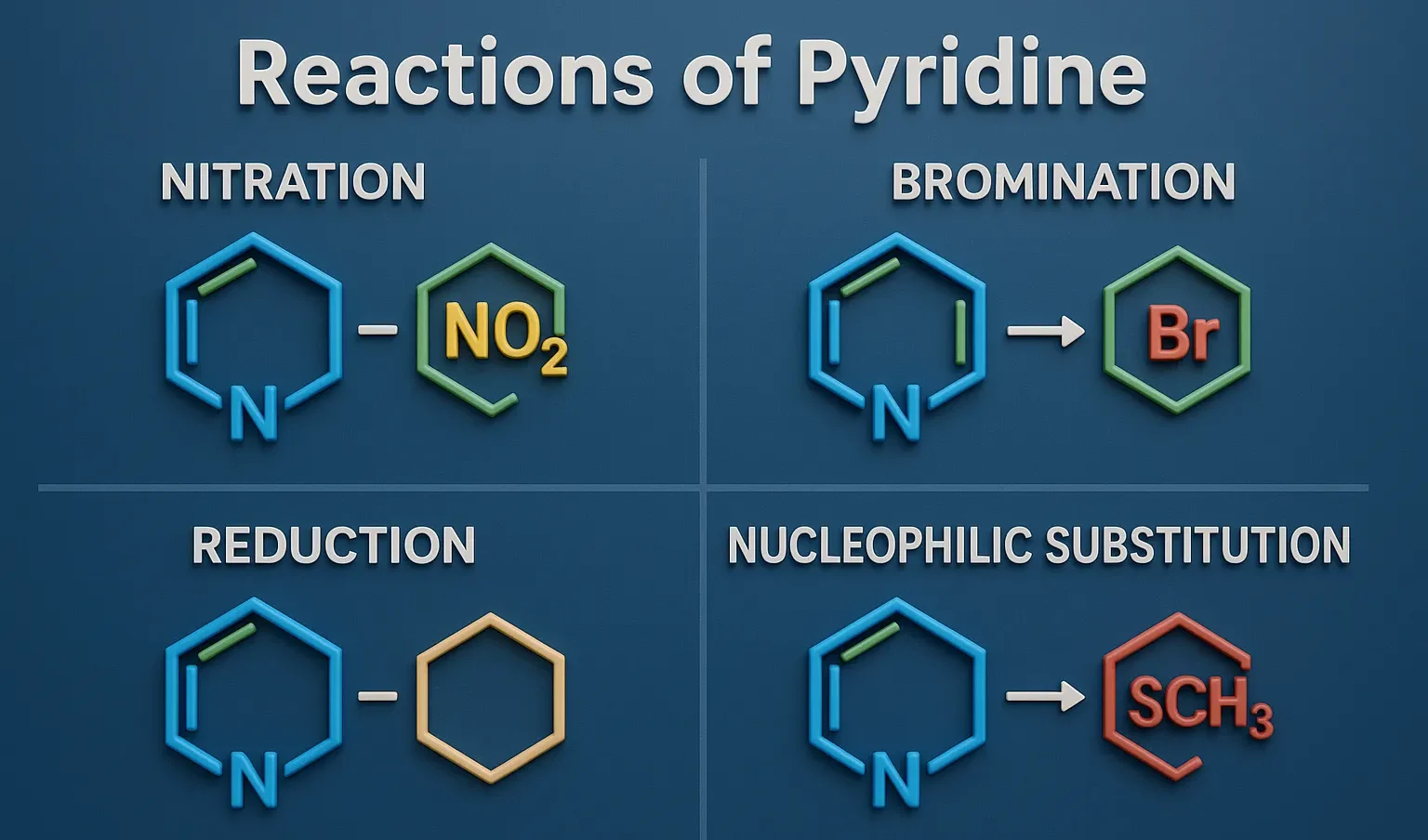
Reactions of Pyridine include electrophilic substitution, nucleophilic substitution, oxidation, and reduction important in drug synthesis.
Reactions of Pyridine
-
Electrophilic Aromatic Substitution (EAS)
- Due to nitrogen’s electron-withdrawing nature, pyridine is much less reactive than benzene toward EAS.
- Preferred Positions:
- C-3 (meta) is the most reactive site for EAS (less destabilized intermediate).
- Deactivation:
- Protonation or complexation with Lewis acid makes ring even more deactivated.
- Typical Reactions:
- Nitration: Harsh conditions (HNO₃, H₂SO₄) → 3-nitropyridine
- Sulfonation: Fuming H₂SO₄ → 3-sulfonic acid
- Halogenation: Br₂ with FeBr₃ catalyst → 3-bromopyridine (hard)
- Friedel–Crafts: Usually doesn’t work unless nitrogen is blocked.
-
Nucleophilic Aromatic Substitution (NAS)
- Pyridine is very reactive to NAS, especially at C-2 and C-4 (activated positions due to N atom).
- Reactions:
- Chloropyridines + nucleophiles (OH⁻, NH₃, RO⁻)
- Example: 2-chloropyridine + NaOH → 2-hydroxypyridine
- Mechanism:
- SNAr (addition-elimination) via Meisenheimer complex.
-
N-Oxide Formation
- Pyridine reacts with peracids (e.g., m-CPBA) → pyridine N-oxide.
- Why important?
- Increases electron density → activates ring to EAS at ortho and para (C-2, C-4).
-
Reduction and Hydrogenation
- Catalytic hydrogenation (H₂, Pd/C) → piperidine (saturated ring)
- Partial reduction → 1,4-dihydropyridine or 1,2-dihydropyridine with hydride sources.
-
Basic Reactions
- Pyridine acts as a base and nucleophile:
- Forms salts with acids (pyridinium ions)
- Used as a base in organic synthesis (e.g., acylation, esterification).
-
Coordination Chemistry
- Nitrogen lone pair coordinates with transition metals (Ni, Fe, Cu, Zn)
- Found in ligands, enzyme models, organometallic catalysts.
-
Oxidation
- Oxidation with KMnO₄ or H₂O₂ under harsh conditions → ring cleavage or pyridine N-oxide.
Advertisements