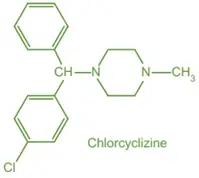
This article explains about the Structure-Activity Relationship (SAR) of H1 receptor antagonist antihistamines and their role in allergy treatment.
Structure-Activity Relationship (SAR) of H1 receptor antagonist antihistamines
- The SAR of H₁ receptor antagonists outlines how their chemical structures influence their ability to block H₁ histamine receptors.
- Key structural components include aromatic substitutions, the linker atom (X), the alkyl chain, and the terminal nitrogen atom.
Basic Structure of Antihistamines
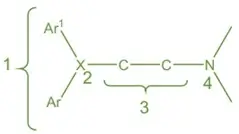
- Aryl Groups: Two aromatic rings are essential.
- Linker Atom (X): Can be Oxygen (O), Carbon (C), or Nitrogen (N).
- Ethylene Bridge: A two-carbon spacer connecting the aryl groups to the amino group.
- Amino Group: Critical for receptor binding.
-
Substitution on Aryl Groups
-
Diary Substitution:
- Essential for Activity: Present in both first and second-generation antihistamines.
- Co-Planarity: Optimal activity requires the two aryl groups to be co-planar.
-
Active Substituents:
- Ar Groups: Phenyl and heteroaryl (e.g., 2-pyridyl).
- Ar₁ Groups: Aryl or aryl methyl.
- Enhancing Substituents: Chlorine (Cl), Bromine (Br), and Methoxy (O-CH₃) groups increase activity.
-
Nature of the Linker Atom (X)
- Common Substitutions: Oxygen (O), Nitrogen (N), Carbon (C).
- Active Linkers:
-
- X = Oxygen: Forms amino alkyl ether analogues.
- X = Nitrogen: Creates ethylene-diamine derivatives, introducing chirality for stereoselective binding.
- X = Carbon: Results in mono amino propyl analogues, maintaining structural integrity.
- Inactive Substitutions: Substituting X with elements other than O, N, or C reduces or abolishes activity.
-
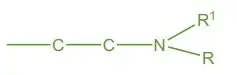
Alkyl Chain
- Ethylene Chain: Essential for activity, providing necessary spacing between functional groups.
- Branching: Reduces activity by disrupting proper alignment and binding efficiency.
-
Terminal Nitrogen Atom
- Tertiary Amine (3° Amine): Maximizes activity by enhancing binding affinity.
- Heterocyclic Incorporation: Incorporating the terminal nitrogen into a heterocyclic ring significantly boosts antihistaminic potency.