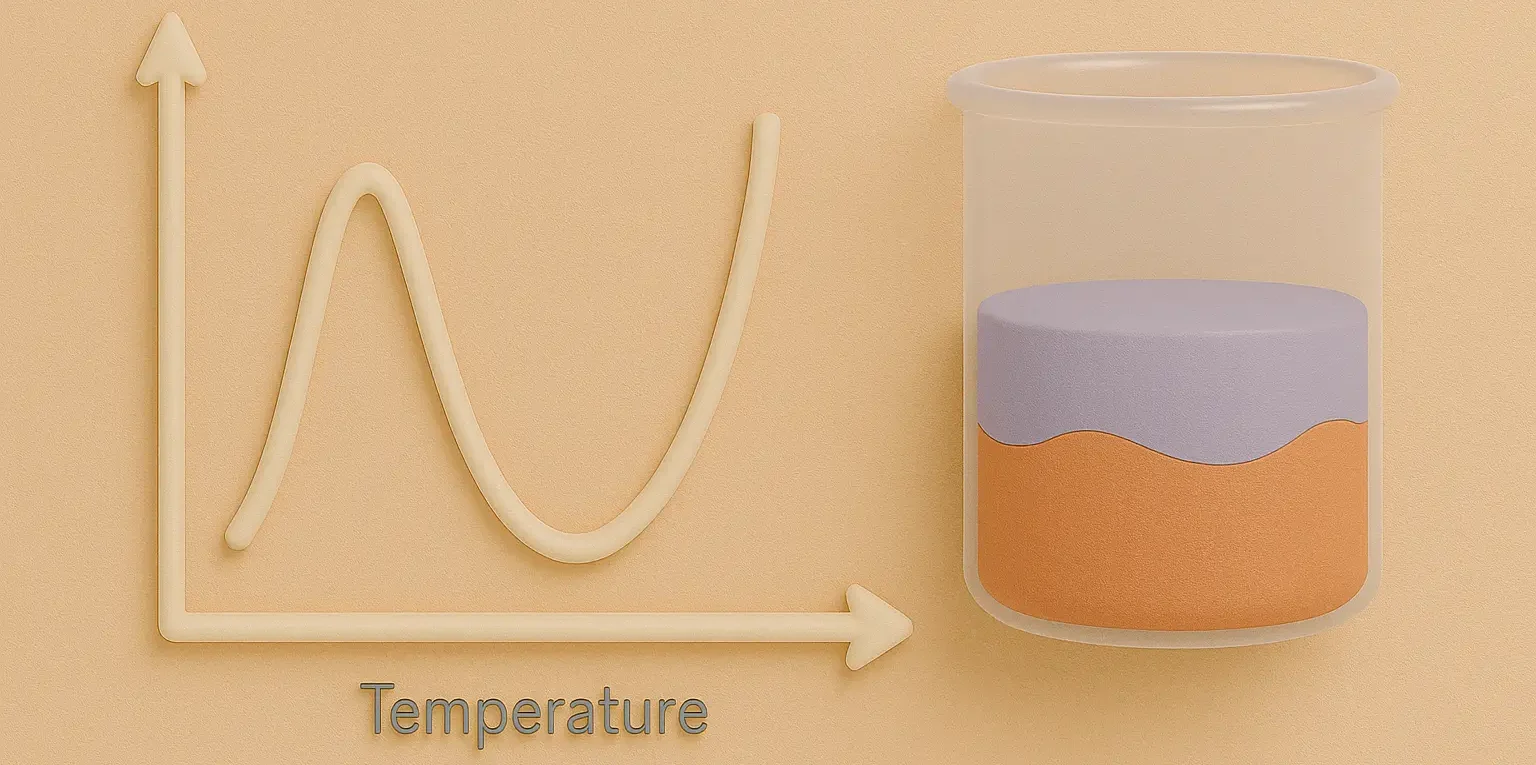
Critical Solution Temperature
Definition: The temperature at which two partially miscible liquids become fully miscible (UCST) or fully immiscible (LCST). Upper Critical Solution Temperature (UCST): Below this temperature, the liquids are completely miscible. Lower Critical Solution Temperature (LCST): Above this temperature, the liquids are completely miscible. Characteristics: Phase Transition Point: Represents the temperature at which the nature of … Read more