Acetazolamide is a carbonic anhydrase inhibitor diuretic used for glaucoma, altitude sickness, and some seizure and edema conditions.
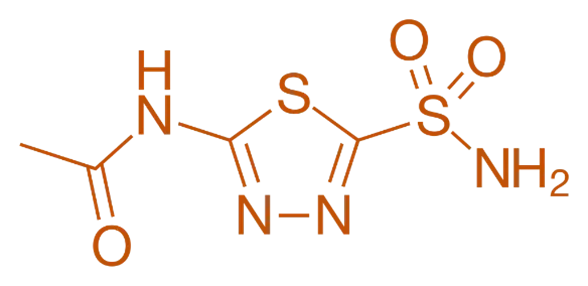
Structure of Acetazolamide
- Acetazolamide is a sulfonamide derivative with a central sulfonamide group attached to a benzene ring and a dithiocarbamate moiety.
- Chemical Formula: C₄H₆N₂O₃S₂
Advertisements
Mode of Action
- Carbonic Anhydrase Inhibition: Acetazolamide inhibits the enzyme carbonic anhydrase, which is pivotal in the reversible hydration of carbon dioxide.
- Renal Effects: In the proximal tubules of the kidneys, this inhibition leads to decreased reabsorption of bicarbonate, resulting in increased excretion of bicarbonate, sodium, potassium, and water.
- Metabolic Acidosis: Causes a mild metabolic acidosis by reducing bicarbonate levels in the blood.
Advertisements
Uses
- Glaucoma: Reduces intraocular pressure by decreasing aqueous humor production.
- Epilepsy: Used as an adjunctive therapy in certain types of seizures.
- Altitude Sickness: Prevents and treats acute mountain sickness by inducing diuresis and metabolic acidosis.
- Metabolic Alkalosis: Corrects metabolic alkalosis by promoting bicarbonate excretion.
- Diuretic: Employed in cases where other diuretics are ineffective.
Structure-Activity Relationship (SAR)
- Sulfonamide Group: Essential for binding to the active site of carbonic anhydrase.
- Dithiocarbamate Moiety: Enhances binding affinity and specificity for the enzyme.
- Benzene Ring: Provides structural stability and facilitates proper orientation for enzyme interaction.
- Substituents: Electron-withdrawing groups on the benzene ring can increase inhibitory potency by enhancing binding interactions.
Advertisements
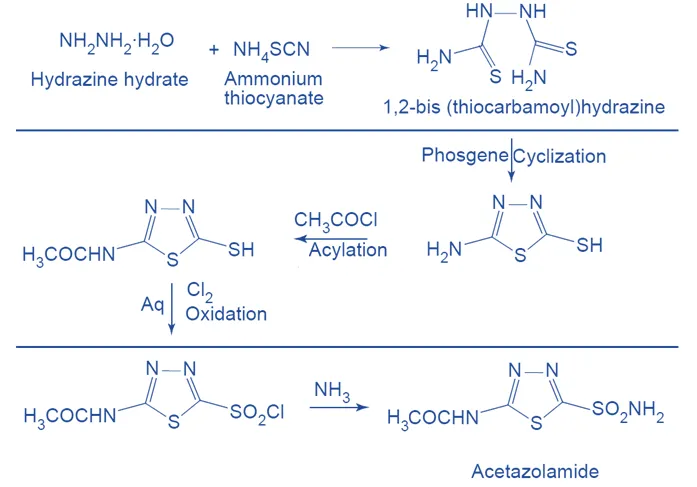
Synthesis