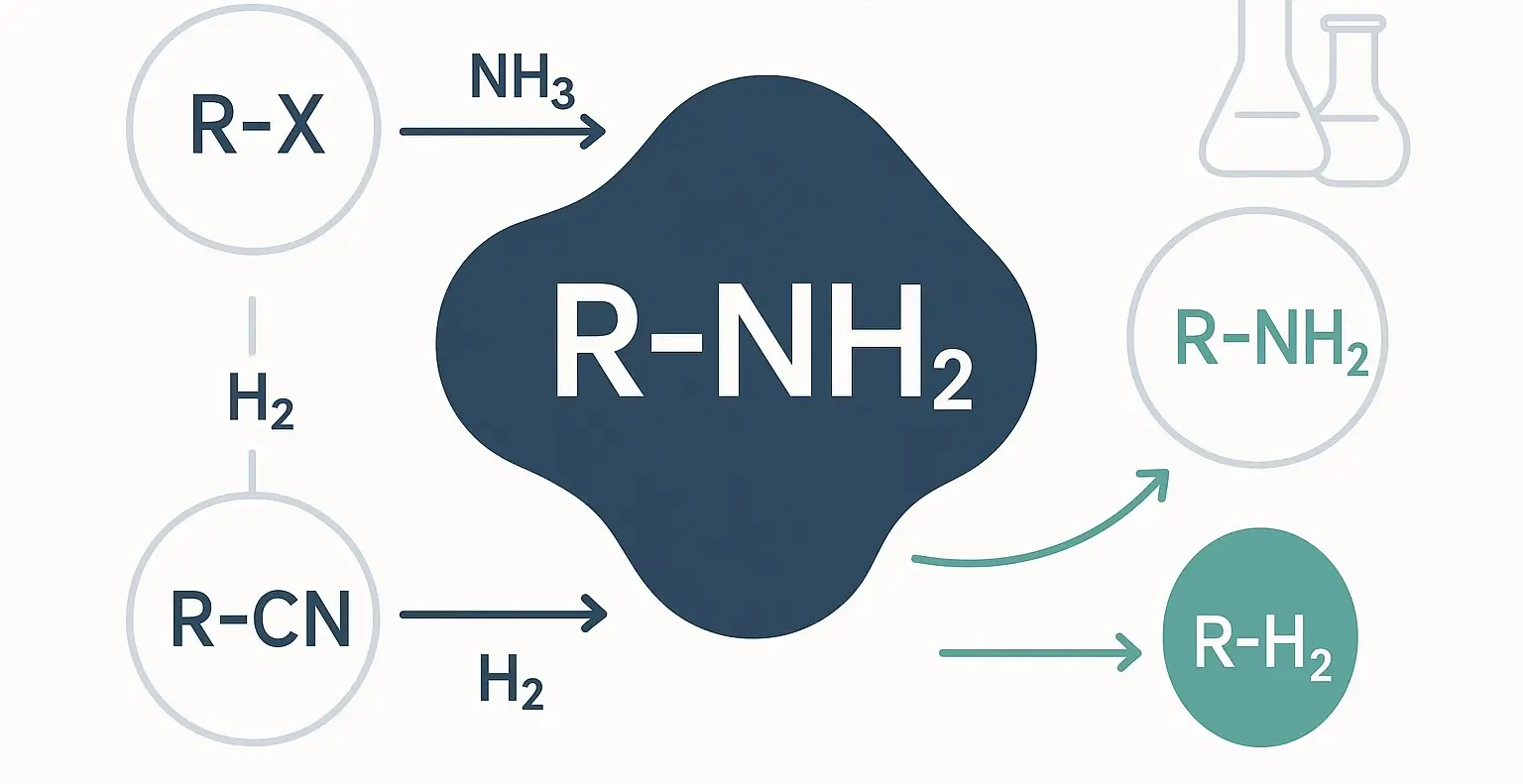
- Aliphatic amines chemical reactions exhibit versatile chemical reactivity due to the nucleophilic nature of the nitrogen atom, which contains a lone pair of electrons.
- Here are some key reactions involving aliphatic amines:
Alkylation as aliphatic amines chemical reactions
-
Process:
- Aliphatic amines react with alkyl halides to form secondary, tertiary amines, and quaternary ammonium salts.
-
Reaction:
- RNH₂ + R’X → RNHR’ + HX
- R and R’ represent alkyl groups, X is a halide ion.
-
Details:
- This reaction follows a nucleophilic substitution mechanism. Over-alkylation can occur if not carefully controlled.
Advertisements
Acylation as aliphatic amines chemical reactions
-
Process:
- Amines react with acid chlorides, anhydrides, or esters to form amides.
- Reaction: RNH₂ + R’COCl → RNHCOR’ + HCl
-
Details:
- The nucleophilic amine attacks the carbonyl carbon of the acylating agent. Acylation is useful for protecting the amine group during synthesis.
Nitrosation
-
Process:
- Primary aliphatic amines react with nitrous acid (HNO₂) to form diazonium salts at 0 to 5°C.
-
Reaction:
- RNH₂ + HNO₂ → [R−N₂]⁺ + 2H₂O
-
Details:
- These diazonium salts are intermediates for synthetic transformations like azo coupling and the Sandmeyer reaction.
Advertisements
Hofmann Elimination
-
Process:
- Quaternary ammonium salts, when treated with a strong base, undergo elimination to form alkenes.
-
Reaction:
- R₄N⁺X⁻ + OH⁻ → R₃N + H₂O + RX
-
Details:
- Useful for synthesizing alkenes with one less carbon atom than the original amine.
Reaction with Hinsberg’s Reagent
-
Process:
- Amines react with benzene sulfonyl chloride (Hinsberg’s reagent) to form sulfonamides, distinguishing between primary, secondary, and tertiary amines.
- Primary amines: Form N-alkylsulfonamides (soluble in alkali).
- Secondary amines: Form N,N-dialkylsulfonamides (insoluble in alkali).
- Tertiary amines: Do not react.
- Amines react with benzene sulfonyl chloride (Hinsberg’s reagent) to form sulfonamides, distinguishing between primary, secondary, and tertiary amines.
Advertisements
Gabriel Phthalimide Synthesis
-
Process:
- Used to synthesize primary amines by reacting phthalimide with an alkyl halide, followed by hydrolysis.
-
Details:
- Useful for preparing primary amines without over-alkylation.
Nucleophilic Substitution Reactions
-
Process:
- Primary and secondary amines act as nucleophiles in substitution reactions with electrophiles (carbon, sulfur, nitrogen centers).
-
Details:
- This allows for the formation of various derivatives and is essential in organic synthesis.
Electrophilic Aromatic Substitution
-
Process:
- Aromatic amines (like aniline) undergo electrophilic aromatic substitution more readily than benzene.
-
Details:
- The amino group activates the ring, directing substitution to the ortho and para positions.
Advertisements
- These reactions demonstrate the broad chemical behavior of aliphatic amines, highlighting their importance in synthetic organic chemistry.