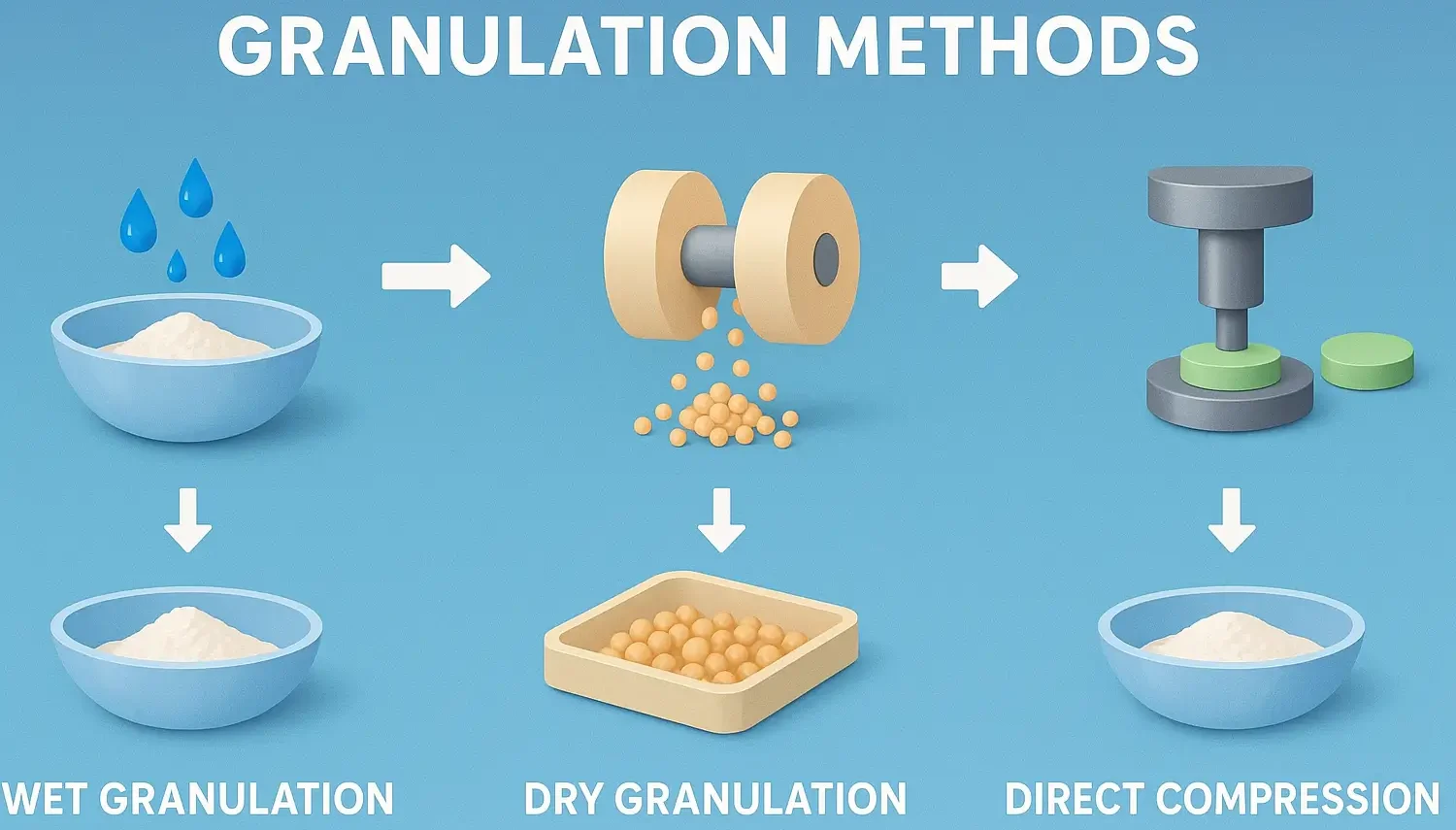
Types of Tablet Coating
Types of Tablet Coating enhance appearance, mask taste, and control drug release profiles. Types of Tablet Coating include sugar coating, film coating, and enteric coating for functional protection. Tablet coating is performed for various reasons: masking taste or odor, improving appearance, protecting the drug from the environment, or modifying drug release. Sugar Coating Traditional method … Read more