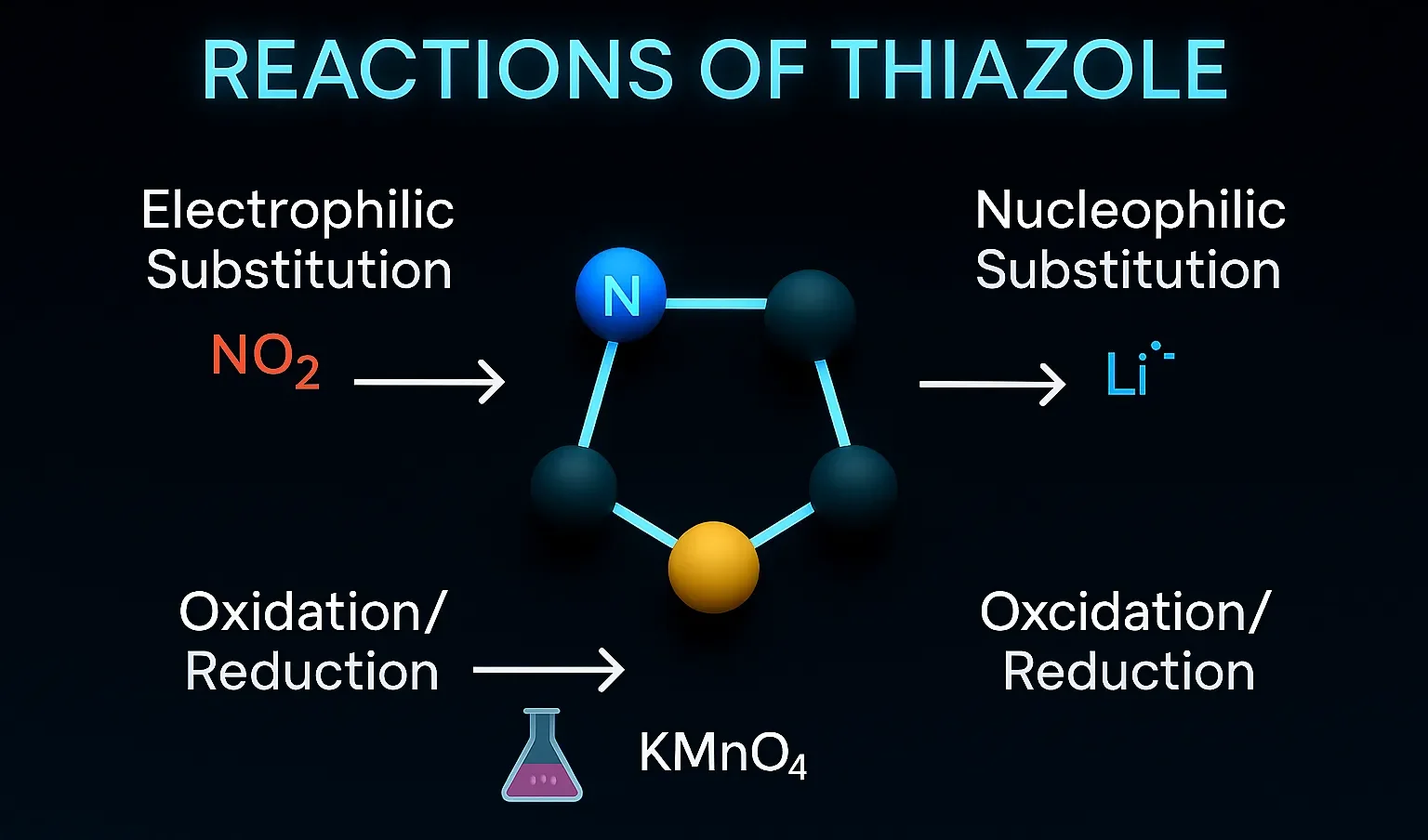
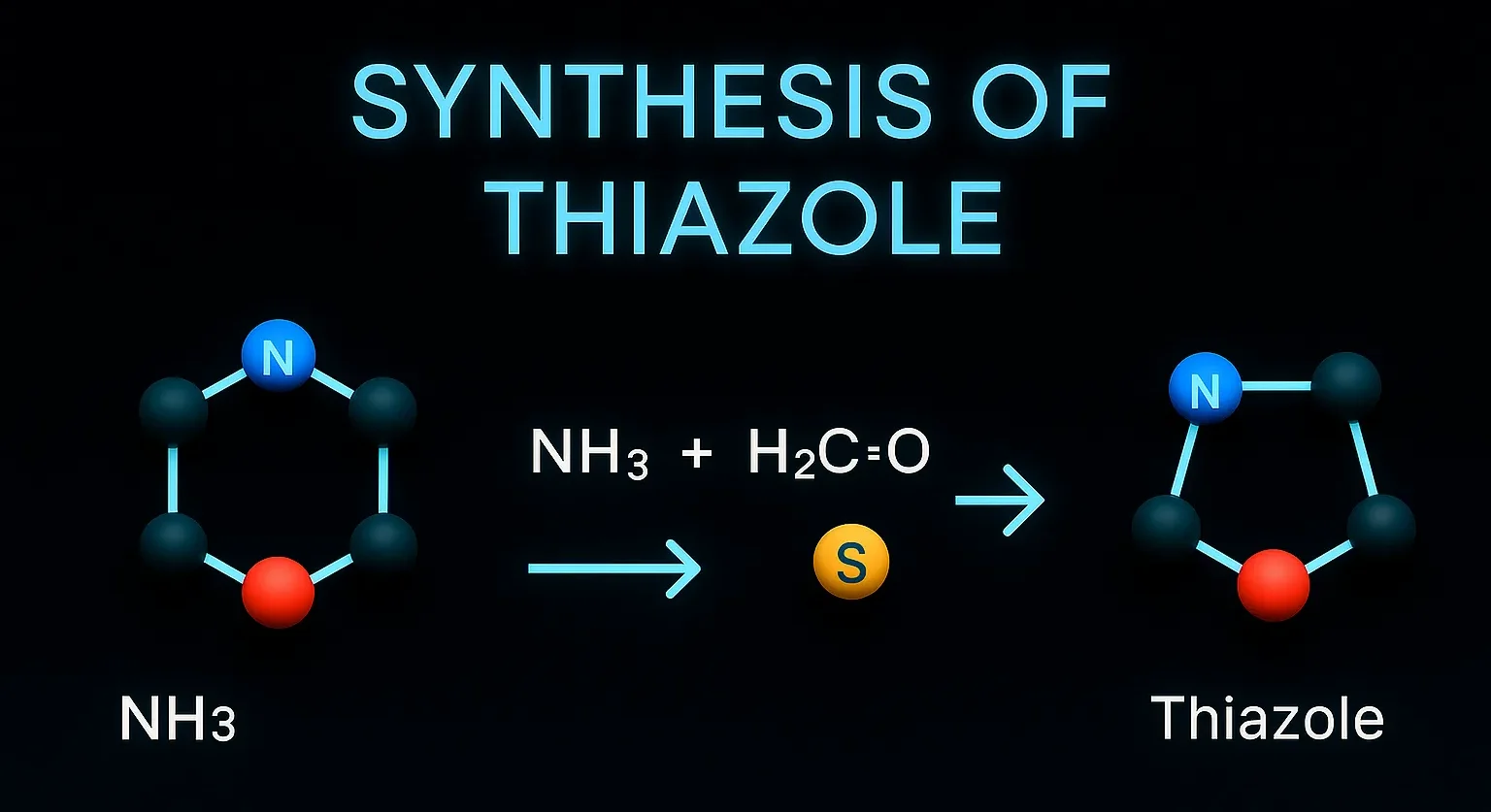
Antipyrine
Antipyrine is used as an analgesic and antipyretic, often in ear preparations for pain relief. It works by inhibiting prostaglandin synthesis, reducing pain, fever, and inflammation. Chemical Formula: C₁₁H₁₂N₂O Mechanism of Antipyrine: COX inhibition Also acts as antipyretic and analgesic Uses of Antipyrine: Historical use for fever, pain Component in ear drop formulations today Side … Read more