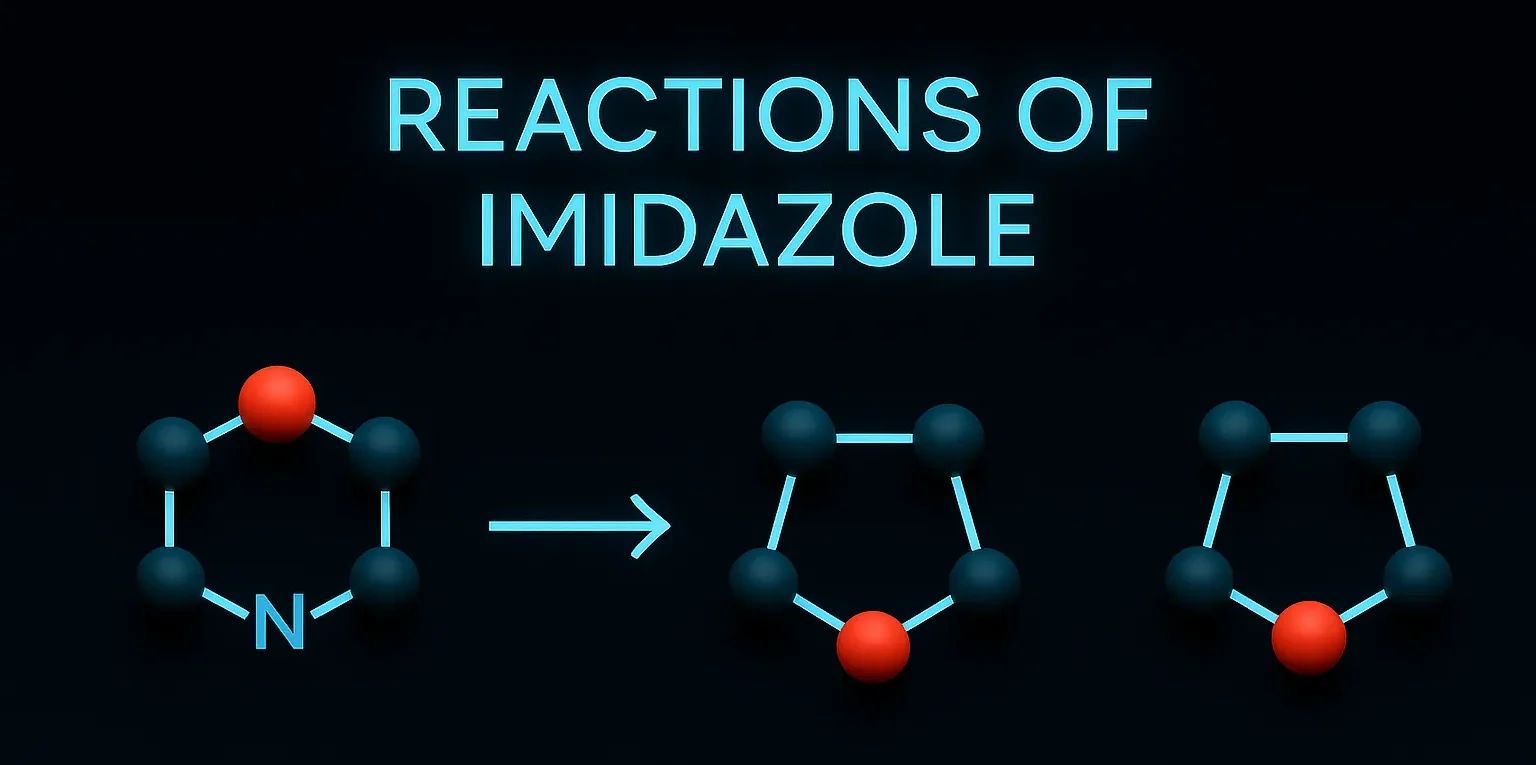
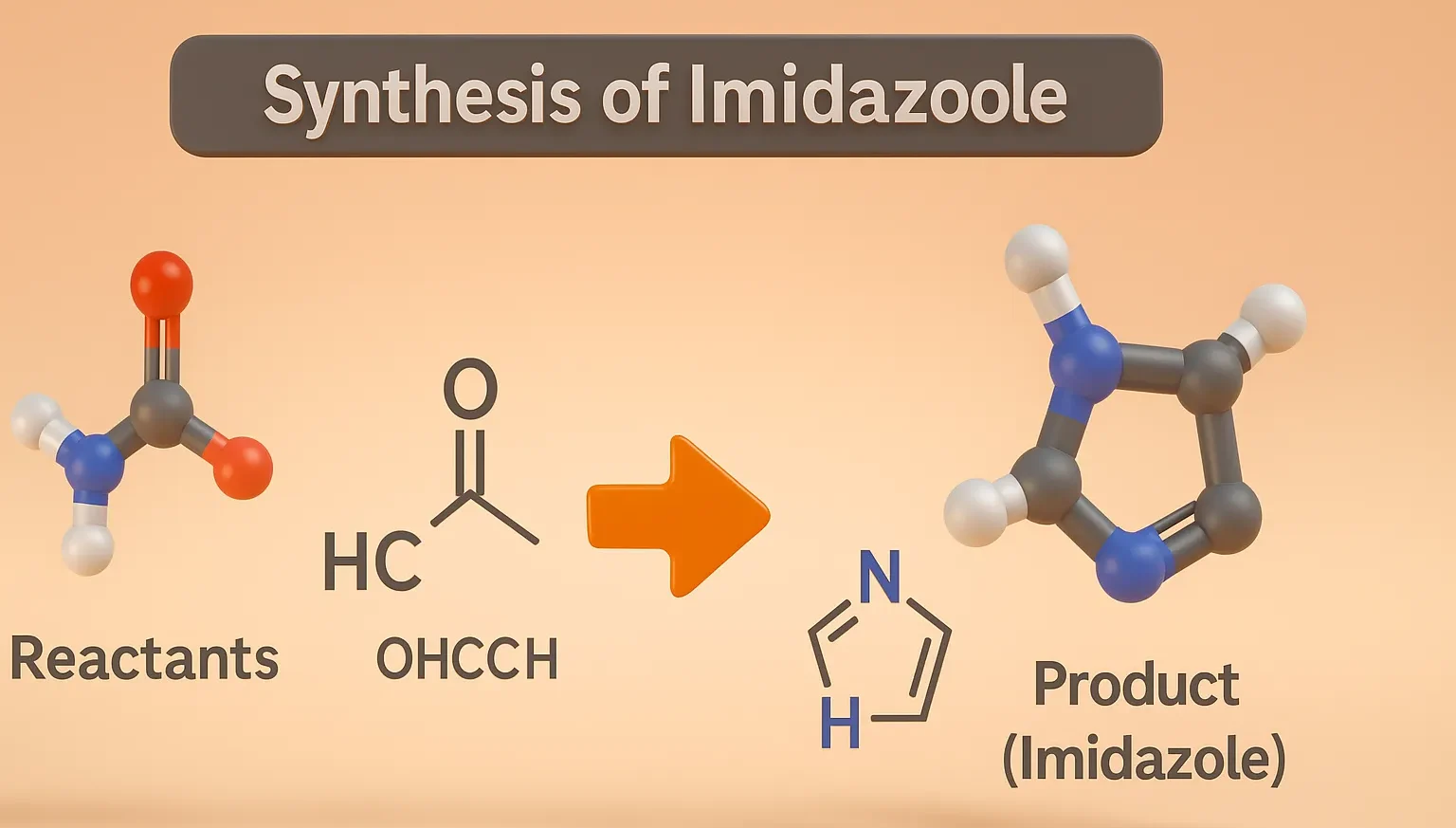
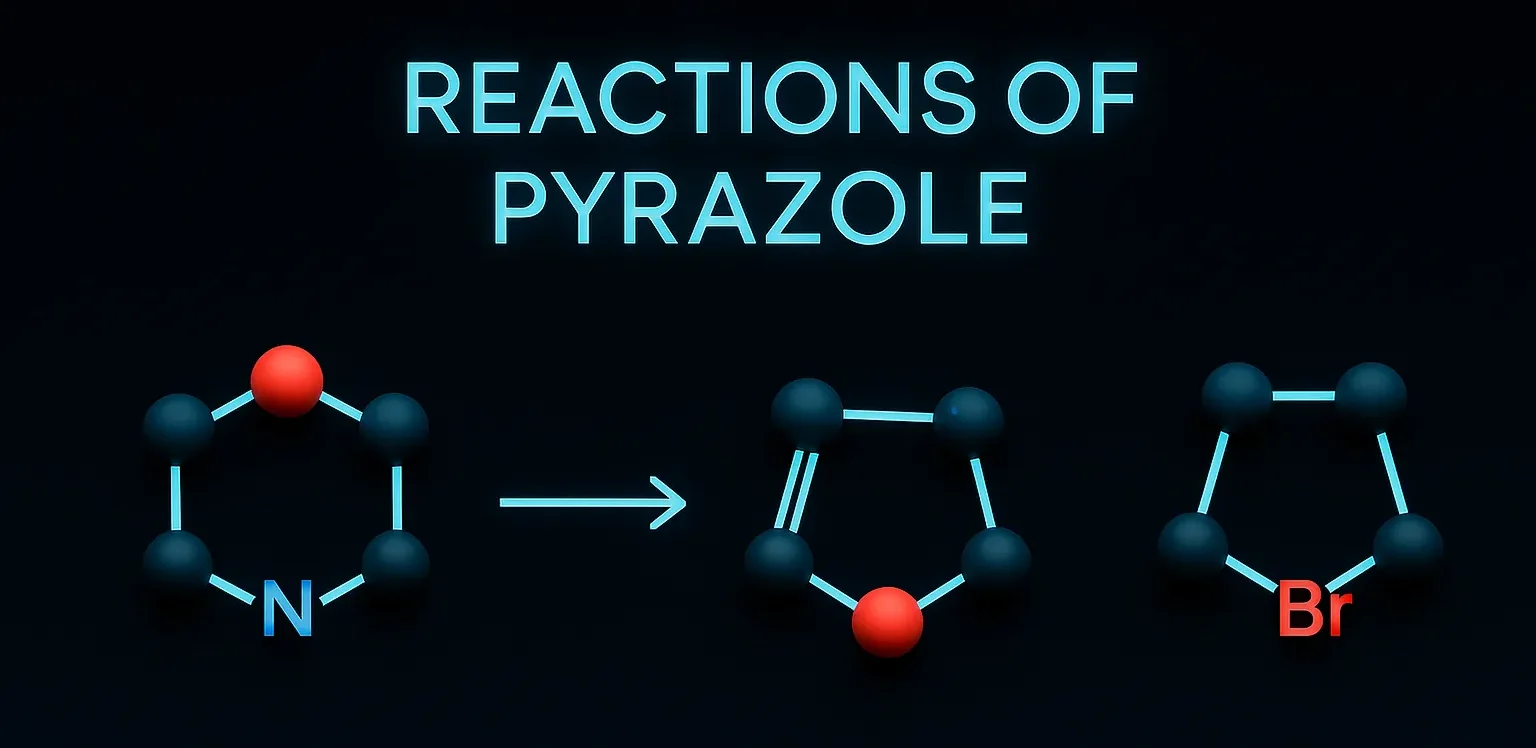
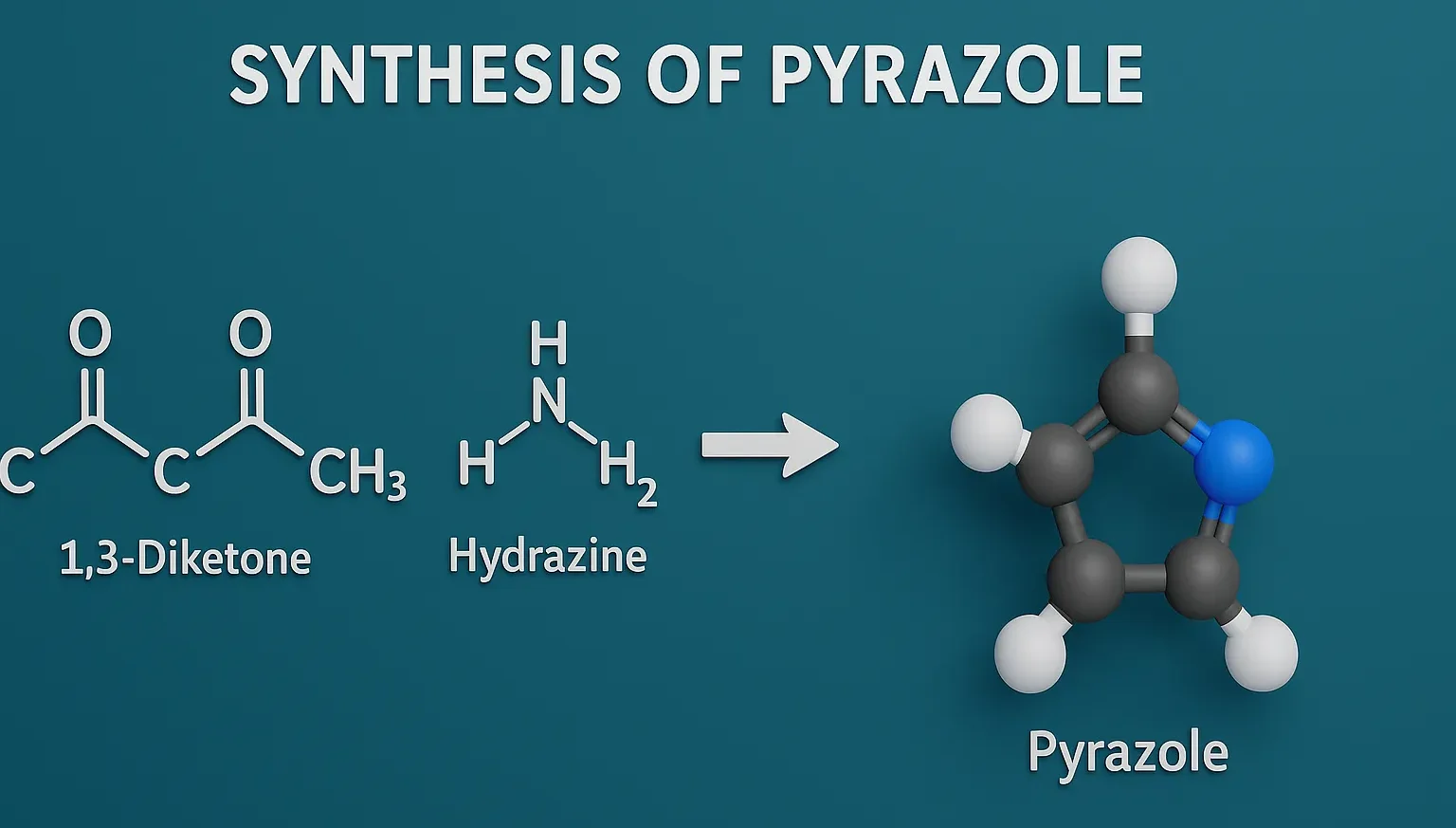
Naproxen
Naproxen blocks COX enzymes, reducing prostaglandins to relieve pain, fever, and swelling. It is an NSAID used for arthritis, muscle pain, menstrual cramps, and inflammation. Chemical Formula: C₁₄H₁₄O₃ Mechanism of Naproxen: Reversible COX-1/COX-2 inhibitor Uses of Naproxen: Arthritis, ankylosing spondylitis Dysmenorrhea Fever, inflammation Side Effects: GI discomfort Cardiovascular risk (less than some NSAIDs) Fluid … Read more