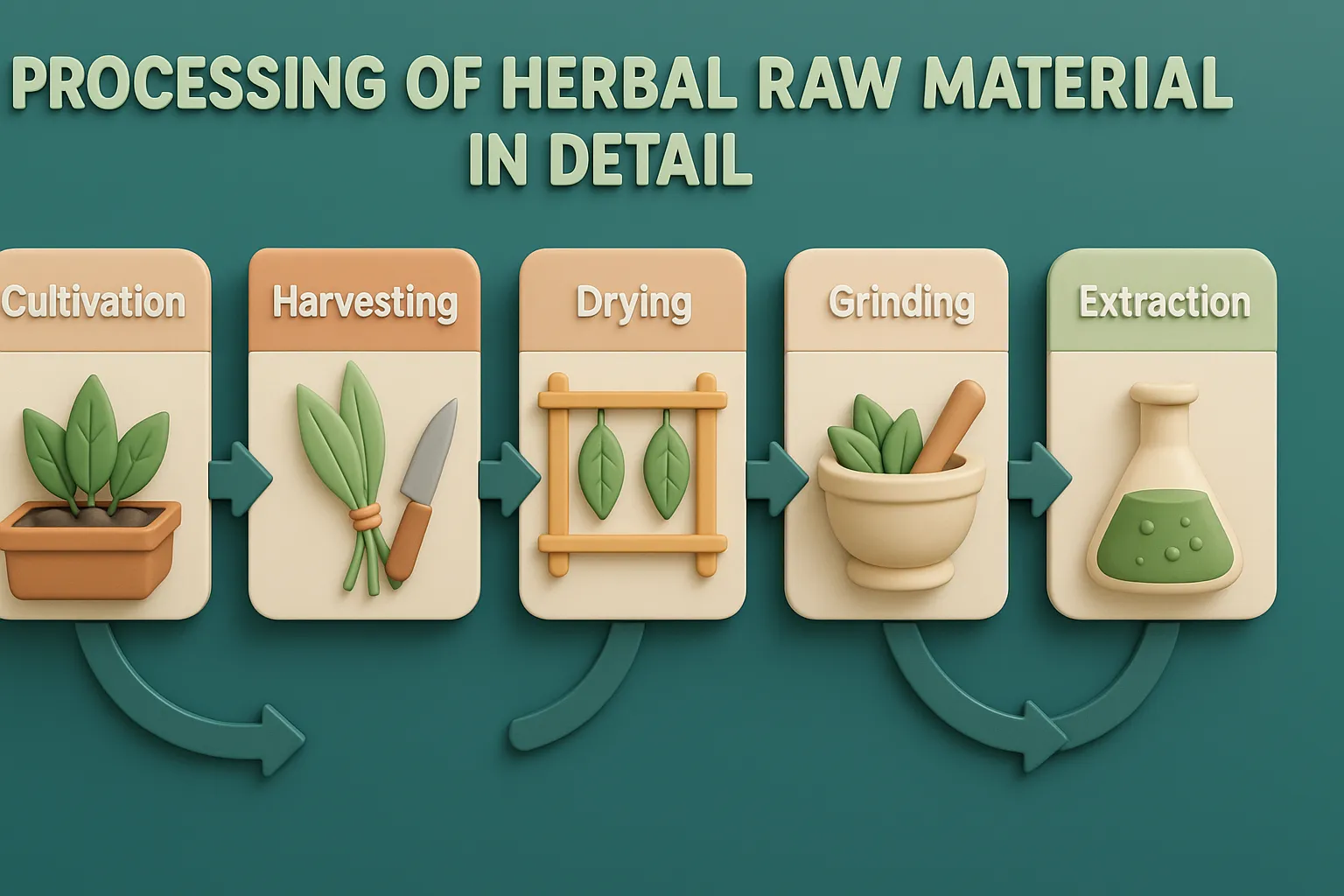
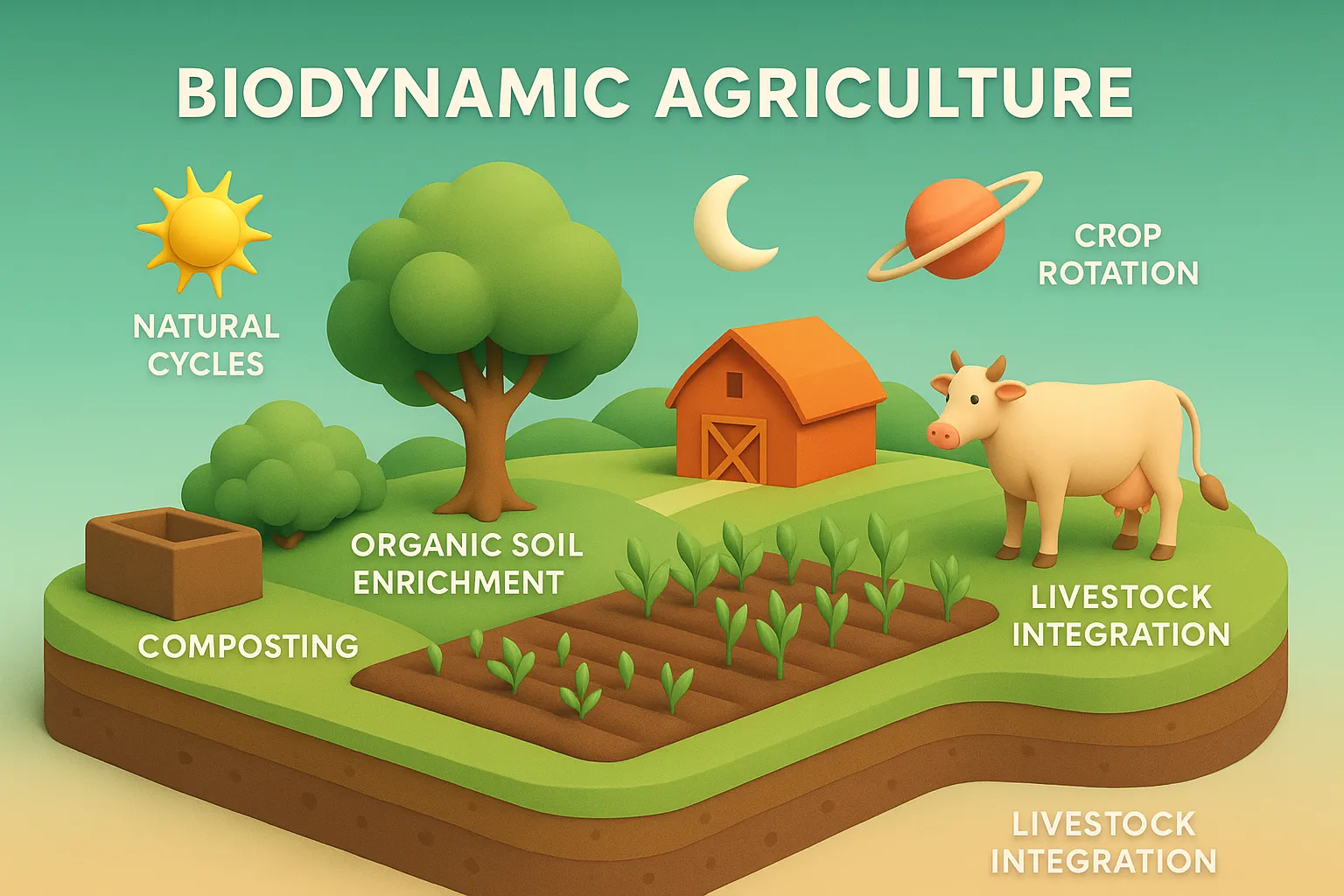
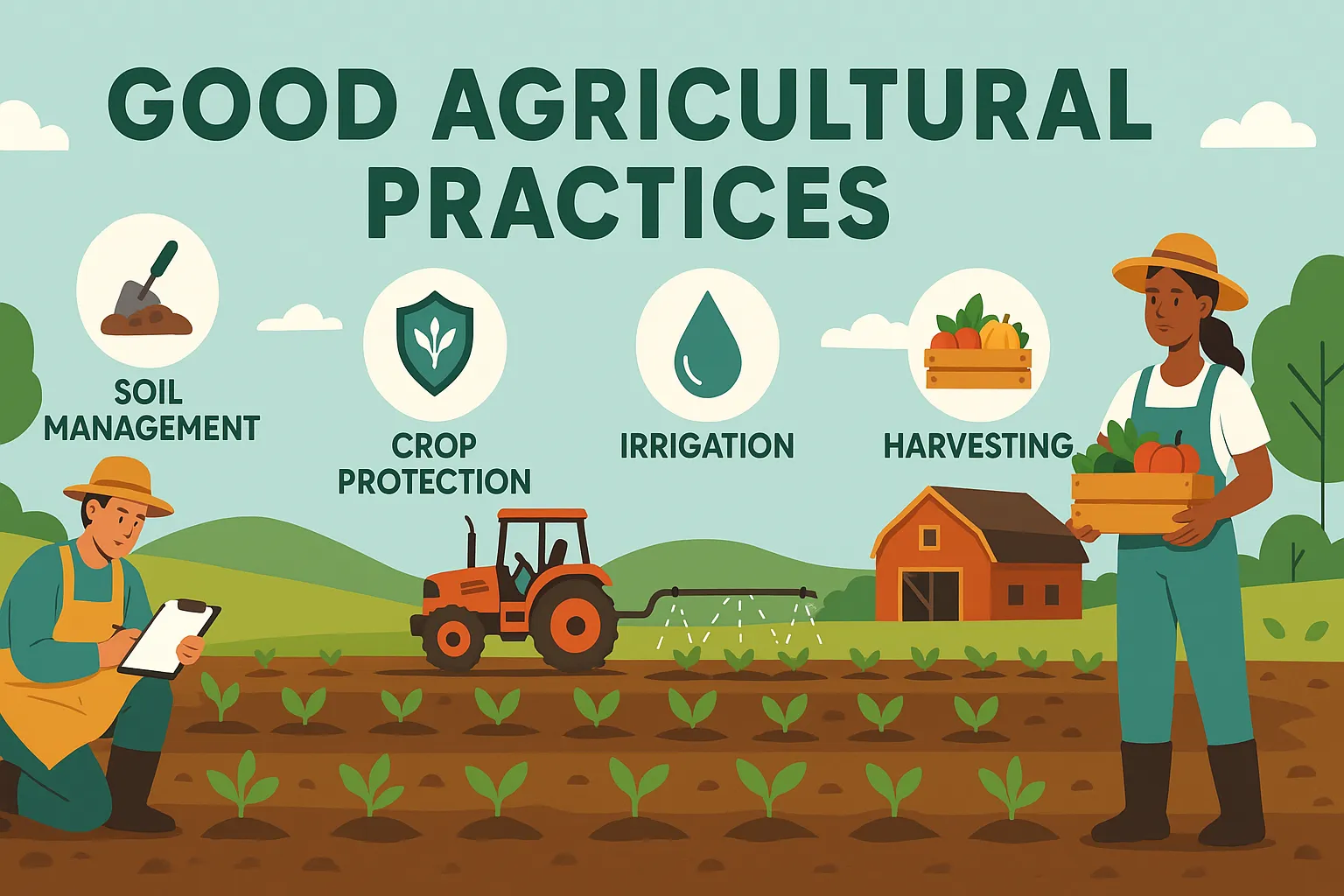
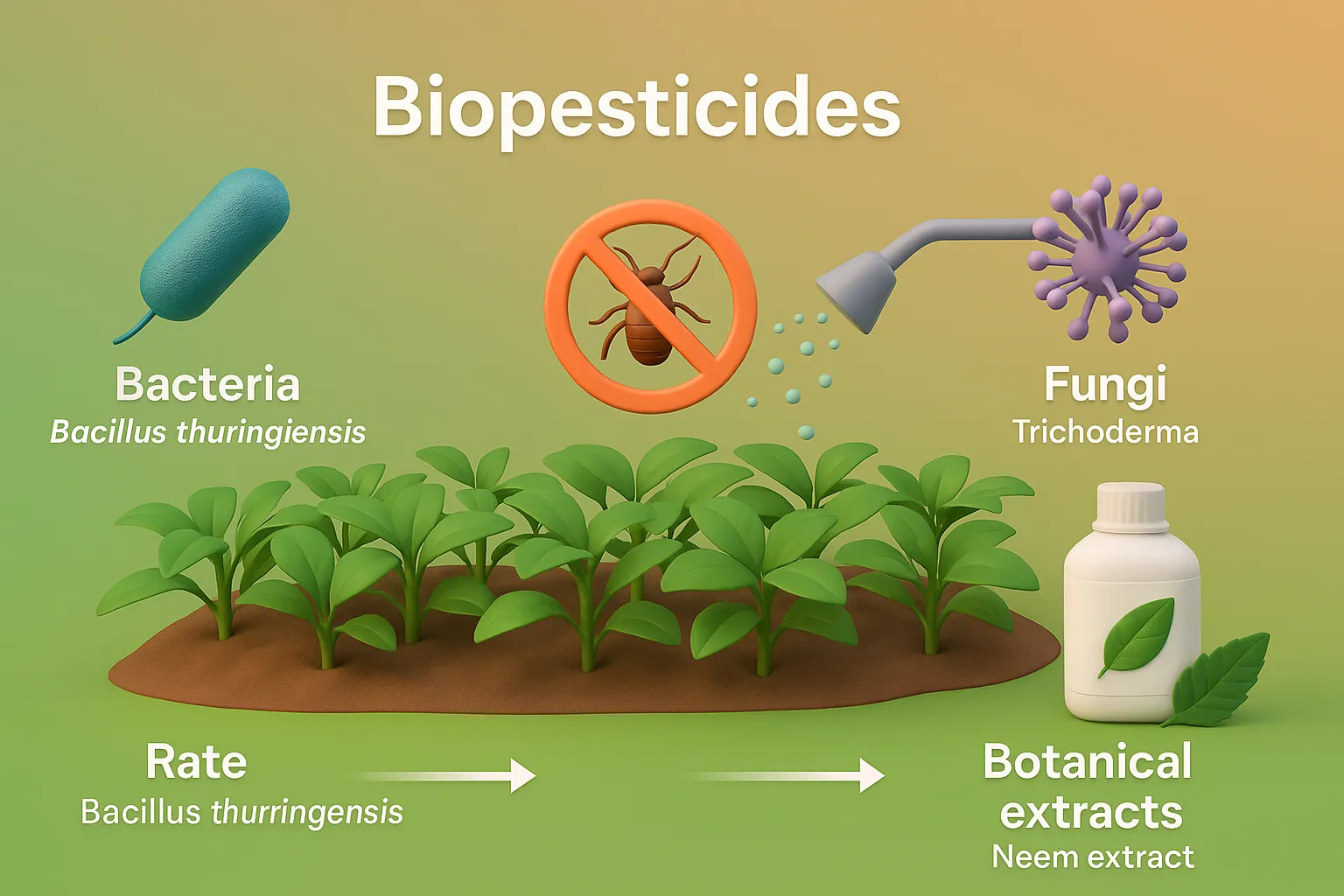
Siddha
Siddha is a traditional South Indian medical system using herbs, minerals, and lifestyle practices to promote health and longevity. Origin and Historical Context Siddha medicine, prevalent in South India (Tamil Nadu), traces its origins to Siddhars—spiritual healers with deep knowledge of medicine, alchemy, and yoga. Key texts include: Theraiyar Yamakam Agathiyar Gunavakadam Yugimuni Siddha Medicine … Read more