- Amino acids are organic molecules that serve as the building blocks of proteins.
- They play essential roles in biological processes, acting as precursors for biomolecules and providing energy under certain conditions.
- Each amino acid consists of:
- Amino group (-NH₂)
- Carboxyl group (-COOH)
- Hydrogen atom (-H)
- Variable side chain (R group) that determines its properties and function.
Classification of Amino acids
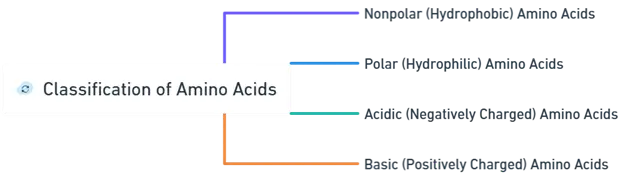
- Amino acids are categorized based on the characteristics of their side chains into four main groups:
- Amino acid is classified based on the chemical properties of their side chains (R groups) into four main groups:
-
Nonpolar (Hydrophobic) Amino Acids
-
Polar (Hydrophilic) Amino Acids
- Properties: Side chains contain functional groups capable of forming hydrogen bonds with water.
- Function: Soluble in water, contribute to hydrophilic interactions in protein structure.
- Examples: Serine, Threonine, Cysteine, Tyrosine, Asparagine, Glutamine.
-
Acidic (Negatively Charged) Amino Acids
- Properties: Contain carboxyl (-COO⁻) groups, making them negatively charged at physiological pH.
- Function: Participate in acid-base interactions and ionic bonding.
- Examples: Aspartic acid, Glutamic acid.
-
Basic (Positively Charged) Amino Acids
- Properties: Contain amino (-NH₃⁺) or positively charged groups, making them positively charged at physiological pH.
- Function: Participate in acid-base interactions and ionic bonding.
- Examples: Lysine, Arginine, Histidine.
Comparison Table: Amino Acid Classification
| Classification | Properties | Function/Interaction | Examples |
| Nonpolar (Hydrophobic) | Mainly C & H, no polar groups | Hydrophobic interactions, found in protein cores | Glycine, Alanine, Valine, Leucine, Isoleucine, Methionine, Proline, Phenylalanine, Tryptophan |
| Polar (Hydrophilic) | Contain functional groups forming hydrogen bonds | Hydrophilic interactions, often found on protein surfaces | Serine, Threonine, Cysteine, Tyrosine, Asparagine, Glutamine |
| Acidic (Negatively Charged) | Contain carboxyl (-COO⁻) groups | Acid-base interactions, ionic bonds | Aspartic acid, Glutamic acid |
| Basic (Positively Charged) | Contain amino (-NH₃⁺) groups | Acid-base interactions, ionic bonds | Lysine, Arginine, Histidine |
Properties of Amino Acids
-
Chirality:
- Most acid exists in L- and D-forms (stereoisomers), but proteins in living organisms are composed of L-amino acid.
-
Zwitterionic Nature:
- At physiological pH, acid exists as zwitterions with both positively charged amino groups and negatively charged carboxyl groups.
Functions of Amino Acids
-
Building Blocks of Proteins:
- Amino acid polymerizes into proteins, which perform structural, enzymatic, and regulatory functions in cells.
-
Precursors for Other Biomolecules:
- Some amino acids serve as precursors for:
- Neurotransmitters (e.g., Tryptophan → Serotonin).
- Hormones (e.g., Tyrosine → Thyroid hormones).
- Nucleotides (e.g., Glutamine → Purines).
- Some amino acids serve as precursors for:
-
Energy Source:
- Under starvation or metabolic stress, acids can be broken down to produce energy.
Examples of Amino Acids
- Glycine: The simplest of all amino acids, found in collagen and involved in synthesizing other biomolecules.
- Lysine: An essential acid important for protein synthesis and critical for tissue growth and repair.
- Tryptophan: An essential amino that serves as a precursor for serotonin (a neurotransmitter) and melatonin (a hormone).
Chemical Nature and Biological Role
- They are characterized by the presence of an amino group, a carboxyl group, a hydrogen atom, and a variable side chain attached to a central alpha carbon.
- This structure forms the foundation for their diverse roles in biology, from providing structural elements to proteins to serving as precursors for various biomolecules and as potential energy sources.
- Through these functions, amino play an indispensable role in the maintenance and regulation of life.