Tetrachloromethane (Carbon Tetrachloride)
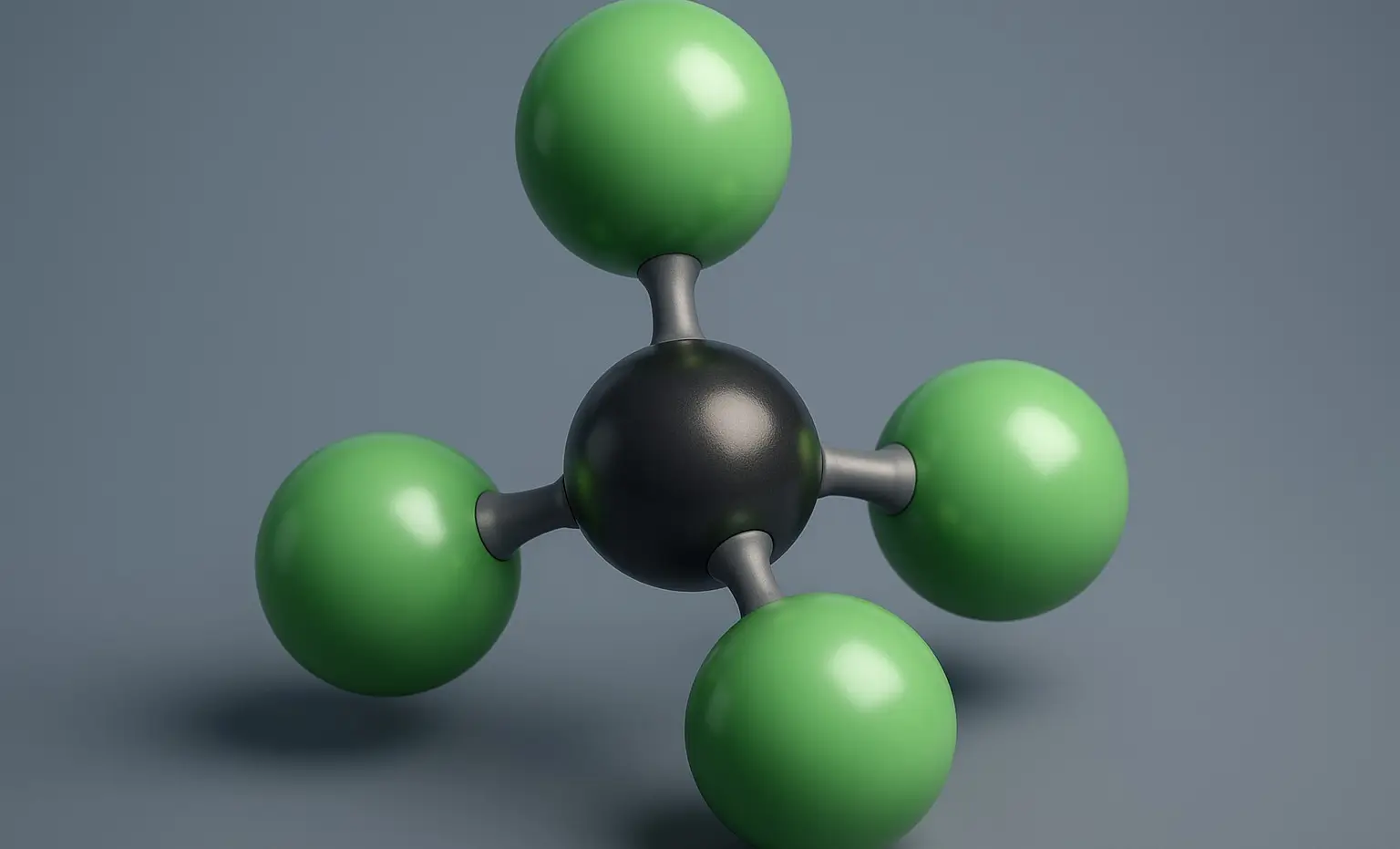
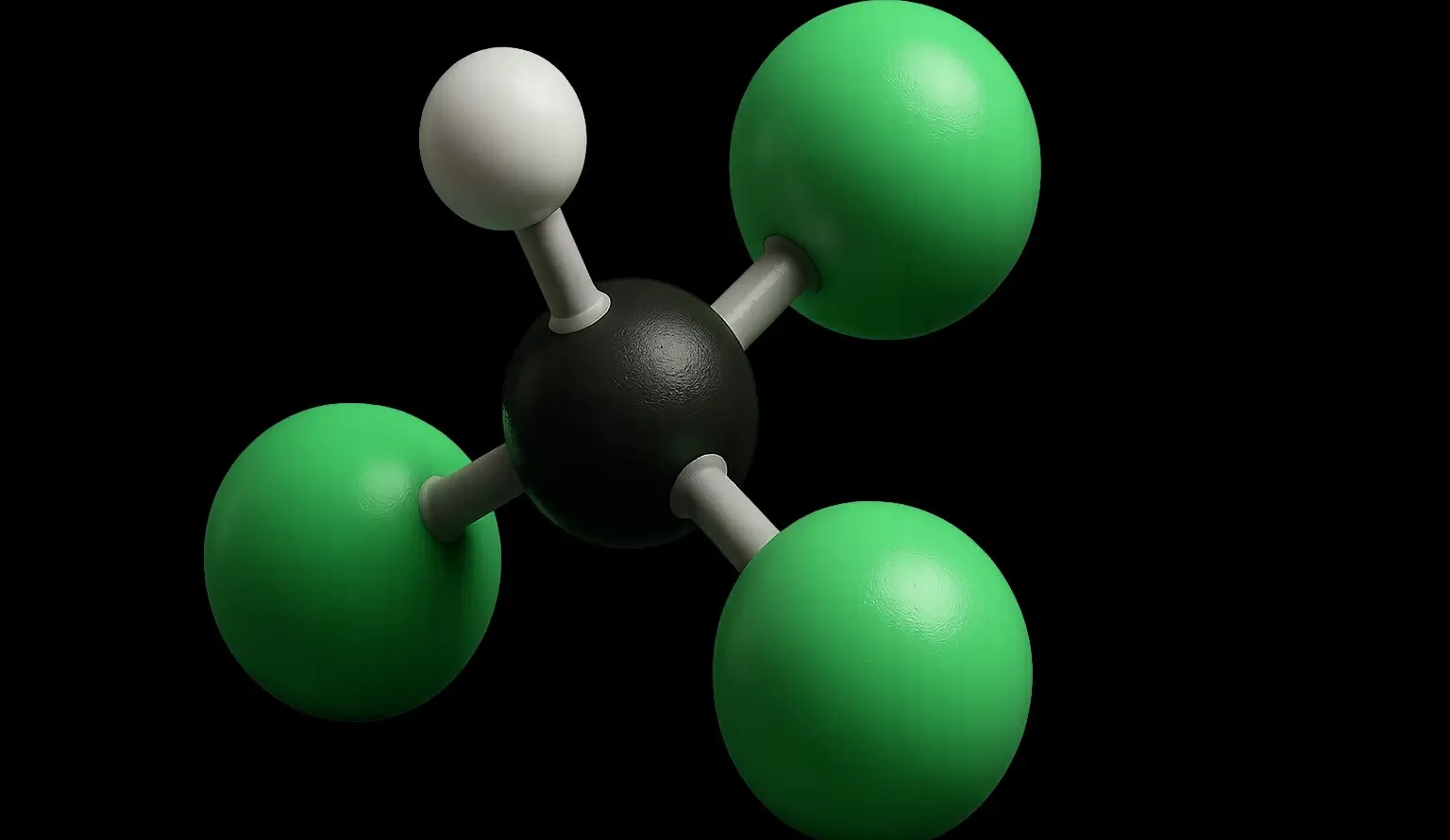
Tetrachloromethane (Carbon Tetrachloride) Definition Tetrachloromethane, commonly known as Carbon Tetrachloride (CCl₄), is a colorless, volatile, non-flammable liquid with a characteristic sweet odor. It is a chlorinated hydrocarbon in which all four hydrogen atoms of methane (CH₄) are replaced by chlorine atoms. Structure: Chemical Formula: CCl₄ Molecular Structure: A single carbon atom bonded to four chlorine … Read more