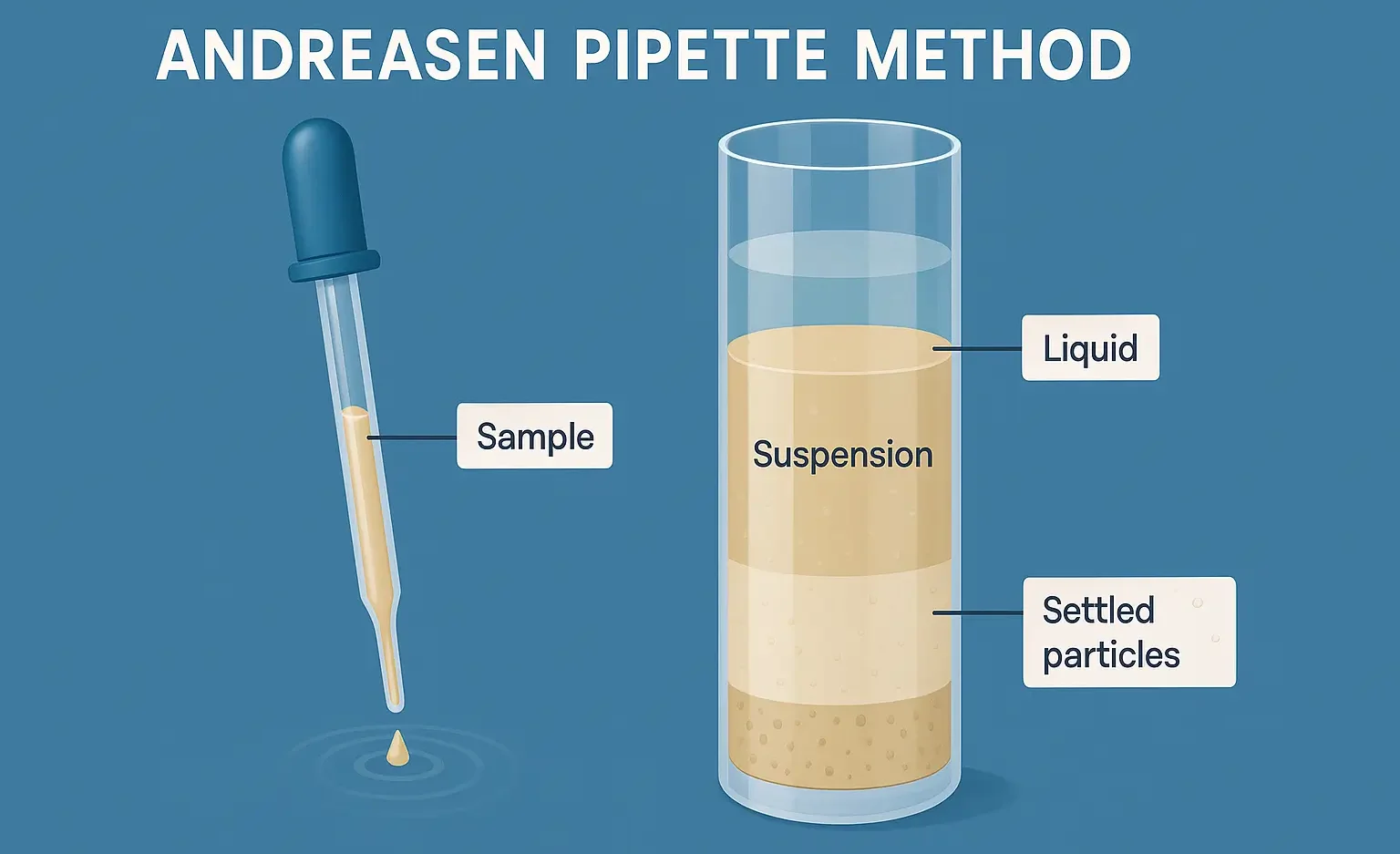
Tissue Permeability of Drugs
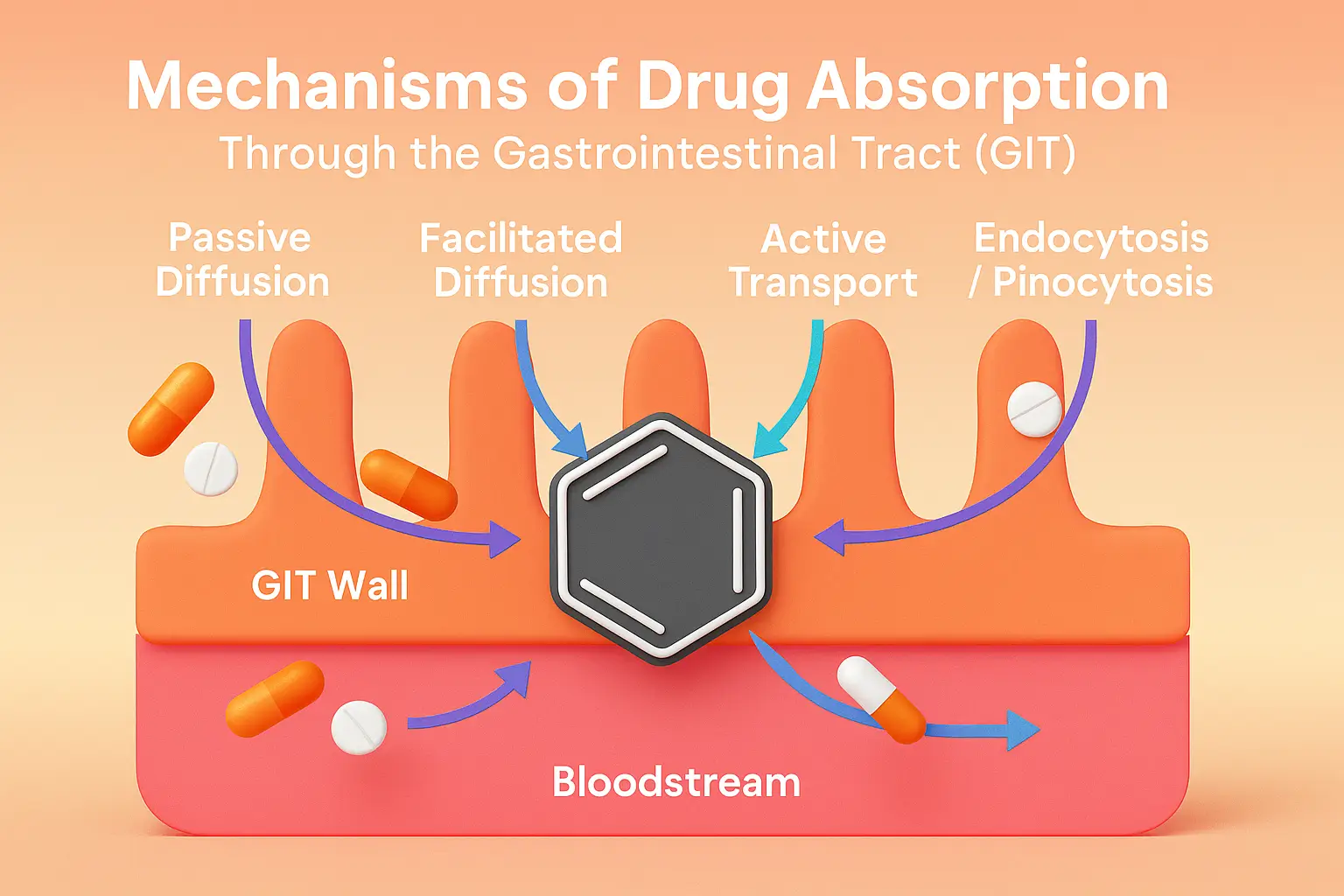
Tissue Permeability of Drugs depends on lipid solubility, ionization, molecular size, and membrane transport mechanisms. Tissue permeability refers to the ability of drugs to cross cell membranes and enter tissues, influencing drug distribution and therapeutic action. It depends on both drug properties and physiological barriers. Factors Influencing Tissue Permeability Lipid Solubility Lipid-soluble drugs easily diffuse … Read more