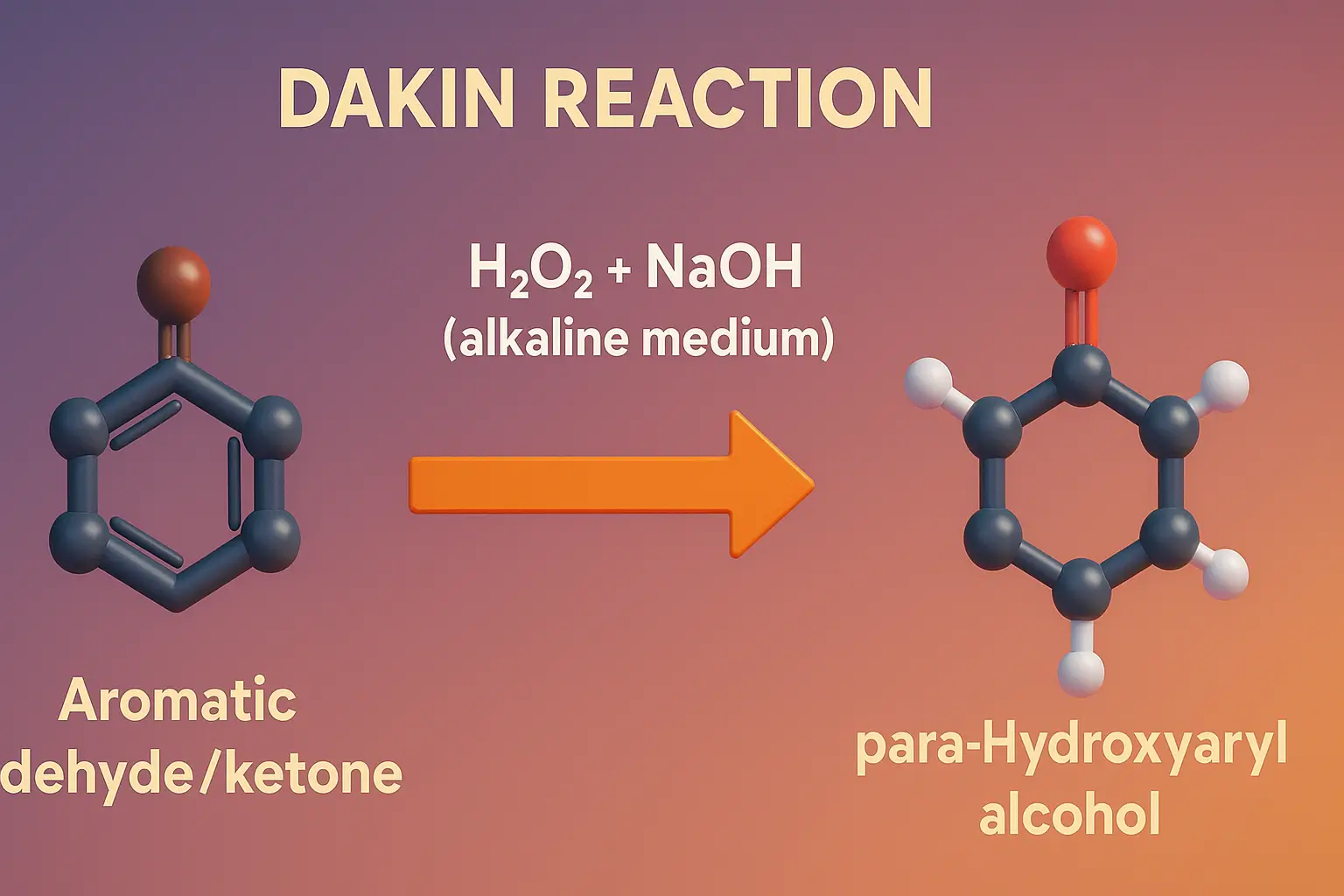
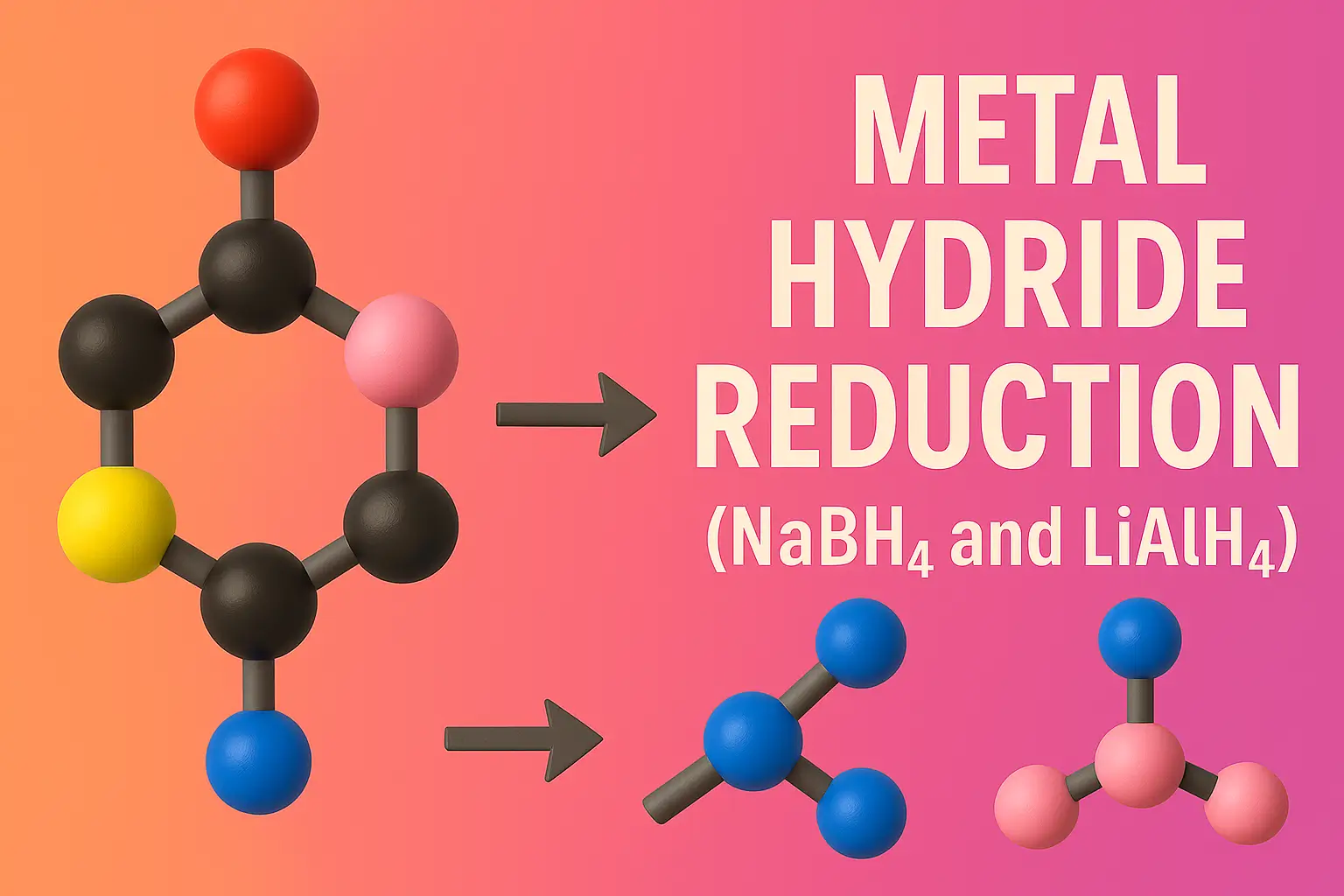
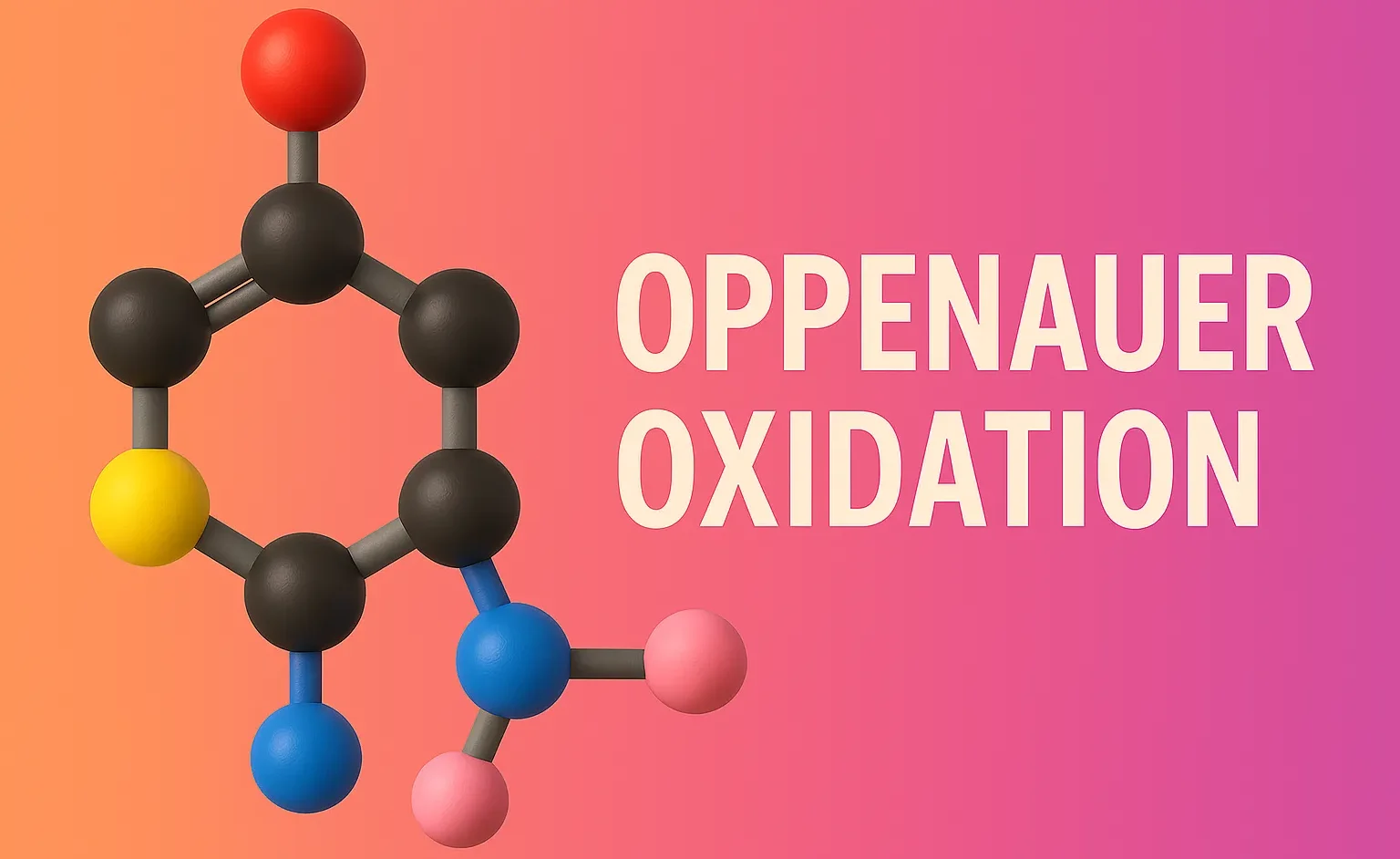
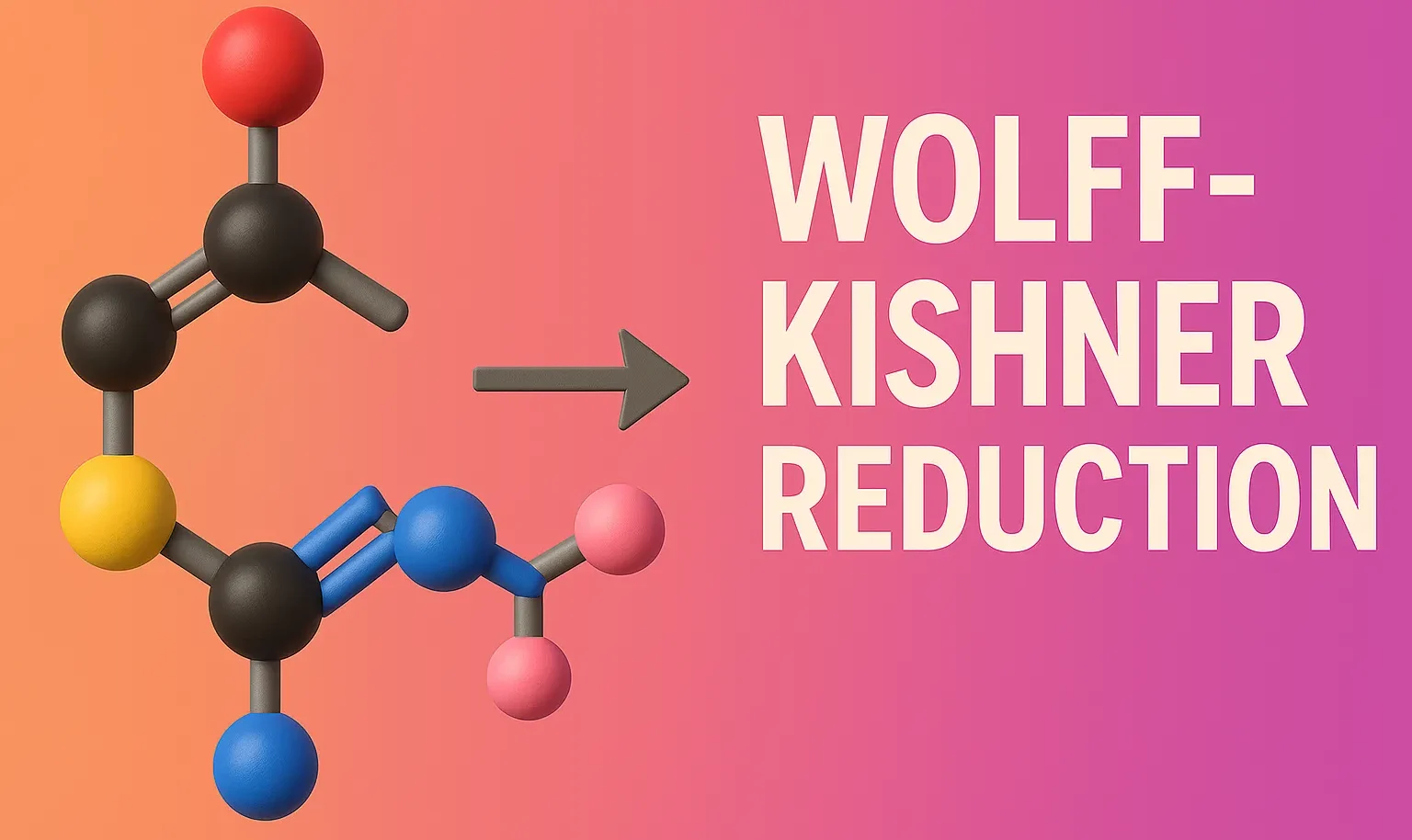
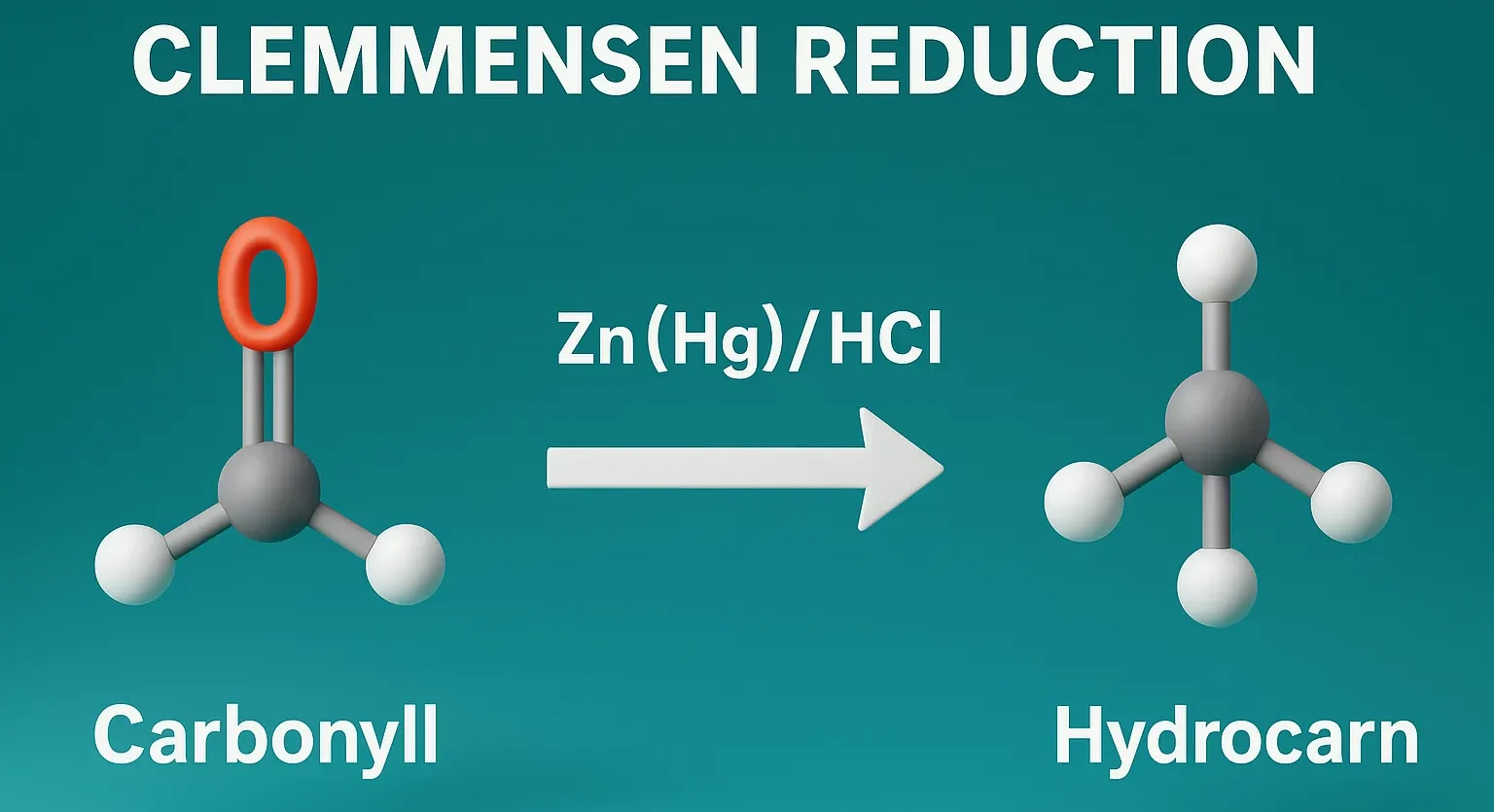
Schmidt Rearrangement
Schmidt Rearrangement converts carboxylic acids, ketones, or aldehydes into amines and amides using hydrazoic acid. The Schmidt rearrangement is a chemical reaction involving the reaction of hydrazoic acid (HN₃) with carbonyl compounds (like carboxylic acids, ketones, or aldehydes) in the presence of acid to yield amines, amides, or nitriles, depending on the substrate. Overview of … Read more