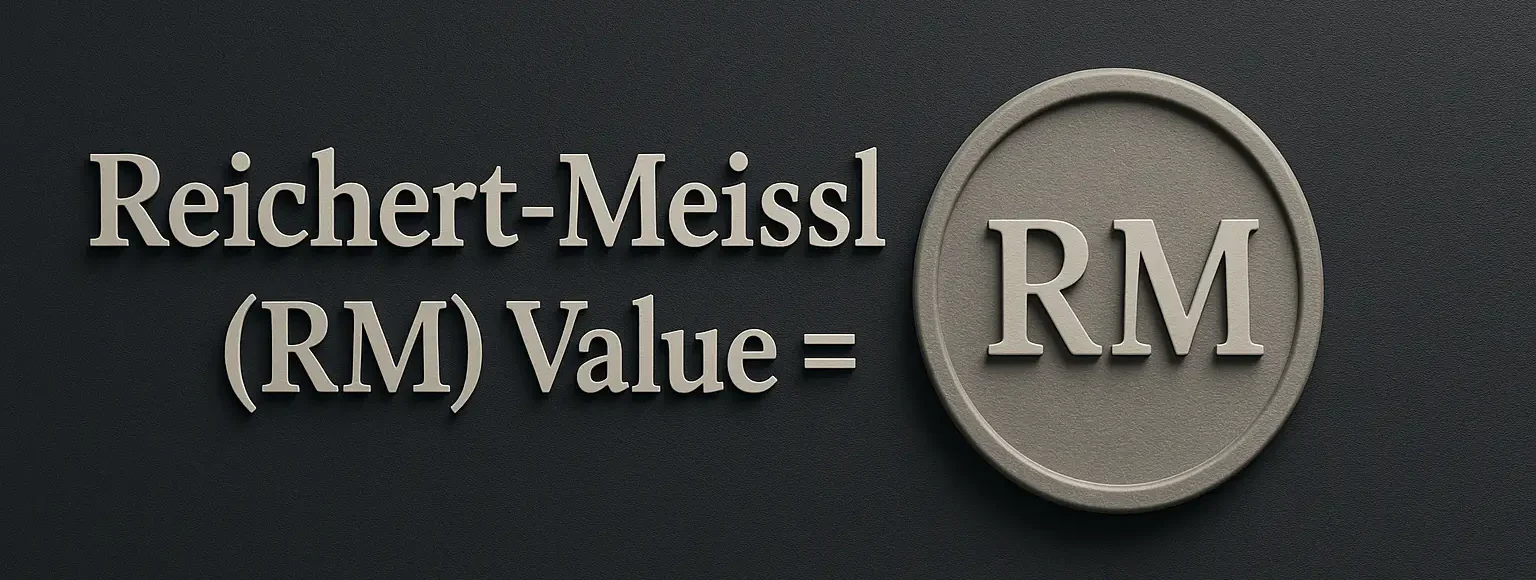Reichert-Meissl (RM) Value
Definition of Reichert-Meissl (RM) Value: The Reichert-Meissl (RM) value is the number of milliliters of 0.1 N potassium hydroxide (KOH) required to neutralize the volatile water-soluble fatty acids distilled from 5 grams of fat or oil. Significance: Indicates the amount of short-chain fatty acids (especially butyric, caproic acids) in the fat, which are typical in … Read more