Azepines are seven-membered heterocyclic compounds important in medicinal chemistry and drug design for CNS and therapeutic agents.
Structure
- These are seven-membered heterocycles with one nitrogen atom.
- Can be saturated or unsaturated (aromatic).
Advertisements
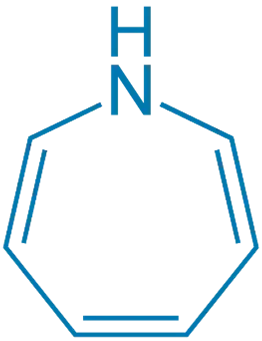
- There are several subclasses:
- Azepine (basic)
- Diazepine (2 N atoms – as in benzodiazepines)
- Thiazepine (N and S atoms)
Advertisements
Advertisements
Synthesis of Azepines
-
Ring Expansion
- From six-membered rings (like cyclohexanones or pyridines) via:
- Rearrangement reactions
- Radical cyclization
- Photochemical methods
- From six-membered rings (like cyclohexanones or pyridines) via:
-
Cyclization Reactions
- Nucleophilic amines with dihaloalkanes.
- Grignard or Wittig reactions to close the ring.
-
Fischer Indole Reaction Variant
- Used to synthesize diazepines and related systems.
Medicinal Uses of Azepines and Derivatives
- Benzodiazepines (e.g., diazepam, lorazepam):
- Act on GABA-A receptors, CNS depressants.
- Used as anxiolytics, sedatives, anticonvulsants.
- Thiazepines:
- Quetiapine – antipsychotic, used in schizophrenia.
- Zotepine – similar mechanism.
- Oxazepines:
- Carbamazepine – anticonvulsant, mood stabilizer.
Advertisements