Beckmann Rearrangement converts oximes into amides or lactams under acidic conditions, important in drug and polymer synthesis.
Overview of Beckmann Rearrangement:
- The Beckmann rearrangement converts oximes into amides via acid-catalyzed rearrangement.
- Generally used for converting ketoximes into N-substituted amides.
- If starting from aldoximes, the product is a primary amide.
Advertisements
General Reaction:
- R1–C=NOH–R2 → R1–CONH–R2
- (in presence of acid catalyst)
For example:
Cyclohexanone oxime → ε-caprolactam (precursor to nylon-6)
Advertisements
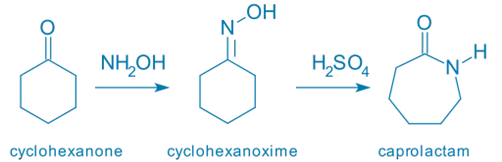
Reagents:
- Acid catalyst: H₂SO₄, HCl, PCl₅, SOCl₂, or polyphosphoric acid (PPA)
- Heat often required
Advertisements
Mechanism (Step-by-step):
-
Step 1: Protonation
- The oxime hydroxyl group is protonated by the acid → activates the molecule for rearrangement.
-
Step 2: Departure of Water
- The –OH₂⁺ group leaves, generating a nitrilium ion (R2C=N⁺R1).
-
Step 3: 1,2-Shift
- The group anti to the OH (i.e., trans to the hydroxyl group in the oxime) migrates to the nitrogen.
- A rearranged nitrilium ion is formed.
-
Step 4: Nucleophilic Attack
- Water (or other nucleophile) attacks the nitrilium carbon.
-
Step 5: Hydrolysis
- Tautomerization/hydrolysis gives the final amide.
Key Features of Beckmann Rearrangement:
- Stereospecific: the group anti to the OH migrates.
- Used in industrial synthesis (e.g., ε-caprolactam for nylon-6).
- Works for both cyclic and acyclic ketoximes.
Click Here to Watch the Best Pharma Videos
Advertisements

