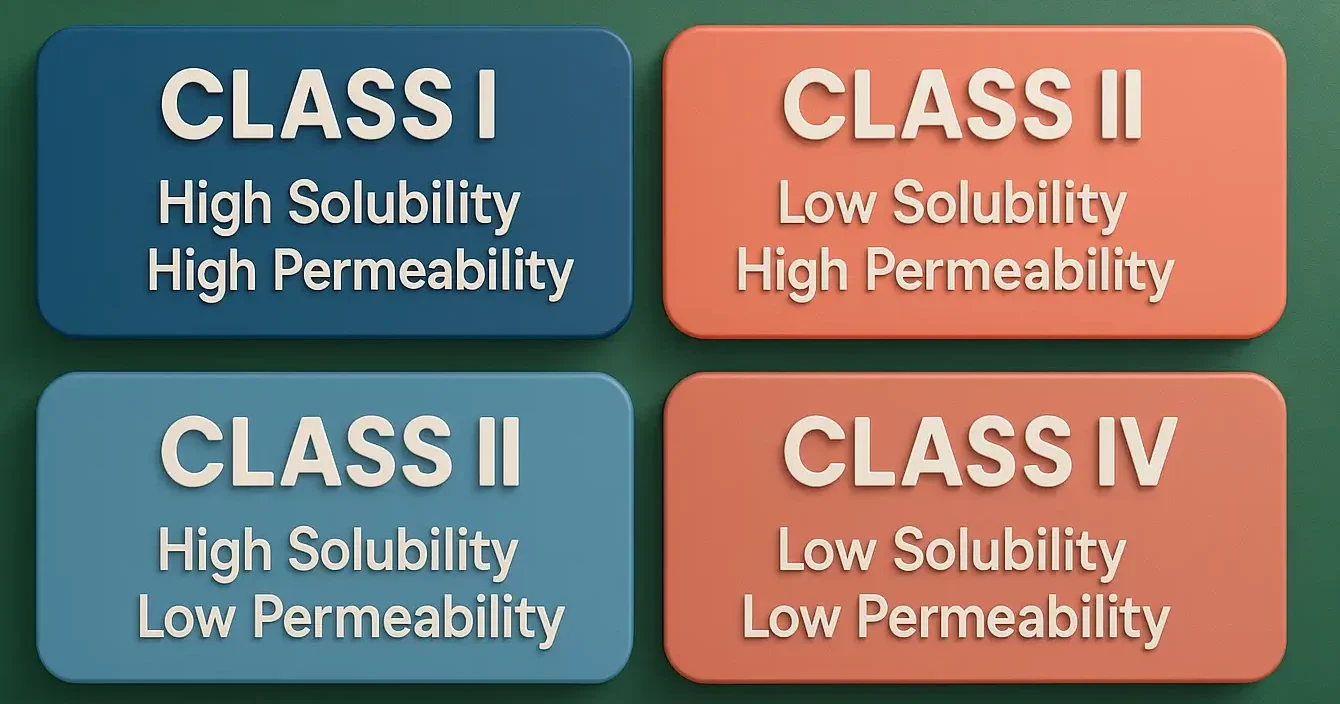
The Biopharmaceutics Classification System (BCS) categorizes drugs based on their solubility and permeability, which influence absorption and bioavailability. It helps in designing formulations and regulatory strategies.
Classification:
| Class | Solubility | Permeability | Examples |
| I | High | High | Metoprolol, Propranolol |
| II | Low | High | Ibuprofen, Ketoprofen |
| III | High | Low | Cimetidine, Ranitidine |
| IV | Low | Low | Furosemide, Hydrochlorothiazide |
Significance of Biopharmaceutics Classification System (BCS):
-
Guides Formulation Strategies:
- Class I: Focus on stability rather than solubility or permeability issues.
- Class II: Enhance solubility through particle size reduction, salt formation, or solid dispersions.
- Class III: Focus on permeability enhancers.
- Class IV: Complex approaches involving both solubility and permeability enhancement.
-
Waiver of Bioequivalence Studies (Biowaivers):
- BCS Class I drugs can receive biowaivers, simplifying generic drug development.
-
Improves Drug Delivery Systems:
- Focuses on enhancing solubility and permeability during formulation.