Introduction to Biphasic Liquids:
- Biphasic liquids are systems consisting of two immiscible (non-mixing) liquid phases.
- These systems are commonly used in the pharmaceutical, cosmetic, and chemical industries.
- They are typically created when two liquids with different polarities, such as oil and water, are combined.
- Due to differences in polarity, these liquids do not mix and form separate layers, creating a biphasic liquid system.
- When left undisturbed, biphasic liquids will naturally separate into their respective layers.
Classification of Biphasic Liquids:
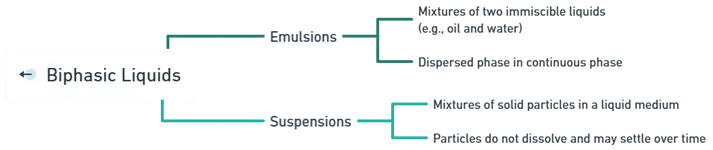
-
Emulsions
- Emulsions are mixtures of two immiscible liquids, such as oil and water, where one liquid (the dispersed phase) is broken down into small droplets and distributed within the other (the continuous phase).
- Emulsions can be further classified into:
- Oil-in-water (O/W) emulsions
- Small droplets of oil are dispersed in an aqueous phase (water-based).
- Water-in-oil (W/O) emulsions
- Small droplets of water are dispersed in an oil phase.
- Multiphase emulsions
- Complex systems with multiple immiscible liquid phases, such as water-in-oil-in-water (W/O/W) or oil-in-water-in-oil (O/W/O) emulsions.
-
Suspensions
- Suspensions are mixtures in which solid particles are dispersed in a liquid medium without dissolving.
- The solid particles remain suspended in the liquid but may settle at the bottom over time if left undisturbed.
- Suspensions are common in pharmaceutical formulations, such as liquid oral medications or topical creams, where the active ingredients are insoluble.
Click Here to Watch the Best Pharma Videos

