Birch Reduction reduces aromatic rings to 1,4-dihydro derivatives using sodium and liquid ammonia in synthesis.
Purpose of Birch Reduction:
- Reduces aromatic rings (like benzene) to non-conjugated cyclohexadienes.
- Partial reduction – breaks aromaticity but doesn’t fully saturate the ring.
Advertisements
Reagents:
- Alkali metal (Na, Li, or K) in liquid ammonia (NH₃)
- Proton source (like ethanol or tert-butanol)
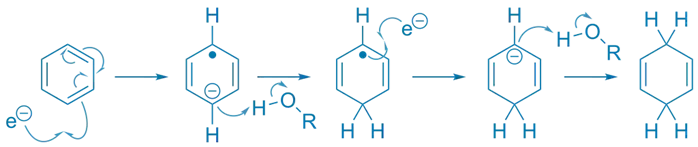
Mechanism Steps (for benzene):
-
Step 1: Electron Addition
- Sodium donates an electron to the aromatic ring → radical anion.
- C₆H₆ + e⁻ → [C₆H₆]⁻•
- Sodium donates an electron to the aromatic ring → radical anion.
-
Step 2: Protonation
- Proton source (ROH) protonates one carbon bearing the radical.
- [C₆H₆]⁻• + ROH → C₆H₅H• (radical)
- Proton source (ROH) protonates one carbon bearing the radical.
-
Step 3: Second Electron Transfer
- Another electron is added → carbanion.
- C₆H₅H• + e⁻ → C₆H₅H⁻
- Another electron is added → carbanion.
-
Step 4: Final Protonation
- Carbanion is protonated again.

- Product: 1,4-cyclohexadiene derivative (with specific regioselectivity).
Advertisements
Example of Birch Reduction:
-
- Benzene → 1,4-cyclohexadiene (with Na/NH₃ and EtOH)
- Toluene → 1,4-cyclohexadiene derivative
Regioselectivity:
- Electron-withdrawing groups (e.g., COOH): reduction occurs at the opposite positions.
- Electron-donating groups (e.g., OCH₃): reduction occurs ortho and para to the group.
Advertisements