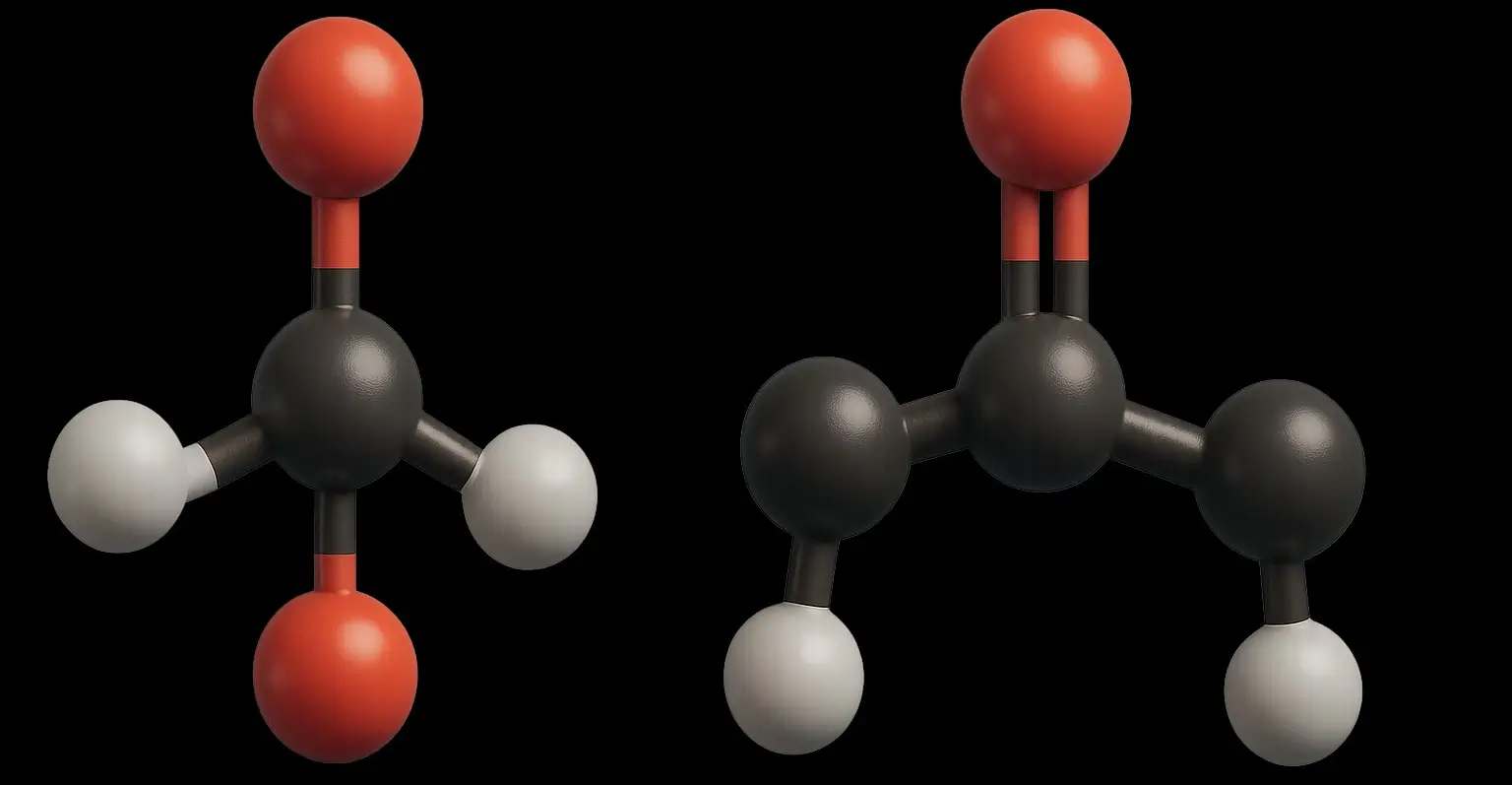
- Carbonyl compounds (Aldehydes and ketones) are a significant class of organic compounds, including aldehydes and ketones, characterized by the presence of a carbonyl group (C=O).
- This group, comprising a carbon atom double bonded to an oxygen atom, contributes to their reactivity and polarity.
- Aldehydes and ketones play vital roles in various industrial and synthetic applications due to their distinctive chemical properties.
Aldehydes
- Aldehydes feature a carbonyl group attached to at least one hydrogen atom and either an alkyl or aryl group.
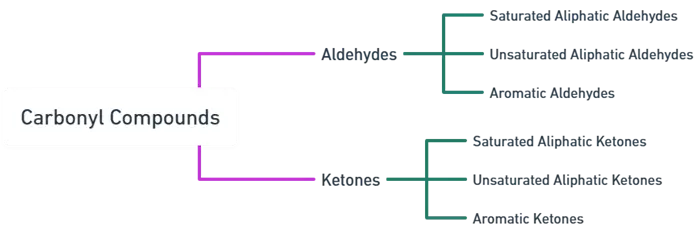
- They are classified based on the nature of the attached carbon chain or ring:
-
Saturated Aliphatic Aldehydes:
- These have a saturated hydrocarbon chain connected to the carbonyl group.
- Examples include formaldehyde (HCHO) and acetaldehyde (CH3CHO).
-
Unsaturated Aliphatic Aldehydes:
- Characterized by one or more double bonds within the hydrocarbon chain attached to the carbonyl group.
- Examples are acrolein (CH2=CHCHO) and crotonaldehyde (CH3CH=CHCHO).
-
Aromatic Aldehydes:
- An aryl group is connected to the carbonyl function.
- Examples include benzaldehyde (C6H5CHO) and cinnamaldehyde (C6H5CH=CHCHO).
Applications of Aldehydes:
- Solvents: Formaldehyde serves as a solvent in some chemical reactions.
- Chemical Synthesis Intermediates: Used in manufacturing plastics, dyes, and perfumes.
- Preservatives: Formaldehyde’s antimicrobial properties make it useful in medical preservation.
- Flavorings and Fragrances: Aromatic aldehydes like cinnamaldehyde and vanillin enhance flavors and scents.
Ketones
- Ketones are characterized by a carbonyl group bonded to two alkyl or aryl groups.
- Their classification is similar to aldehydes but focuses on the nature of the carbonyl-flanking groups:
-
Saturated Aliphatic Ketones:
- These ketones have saturated hydrocarbon chains on both sides of the carbonyl group, with acetone (CH3COCH3) and butanone (CH3CH2COCH3) being common examples.
-
Unsaturated Aliphatic Ketones:
- These contain at least one unsaturated hydrocarbon chain. Methyl vinyl ketone (CH3COCH=CH2) and mesityl oxide (CH3C(O)CH=C(CH3)2) exemplify this category.
-
Aromatic Ketones:
- Here, one or both alkyl groups are replaced with aryl groups. Benzophenone (5C6H5COC6H5) and acetophenone (CH3COC6H5) are notable aromatic ketones.
Applications of Ketones
- Solvents: Acetone and methyl ethyl ketone are key solvents in paints, coatings, and adhesives.
- Organic Synthesis Intermediates: Ketones are pivotal in producing pharmaceuticals, dyes, and polymers.
- Flavorings and Fragrances: Muscone and civetone, for example, are used for their unique scents in the fragrance industry.
- Laboratory Reagents: Ketones serve as essential reagents in Grignard reactions, aldol condensations, and other laboratory processes.
Click Here to Watch the Best Pharma Videos