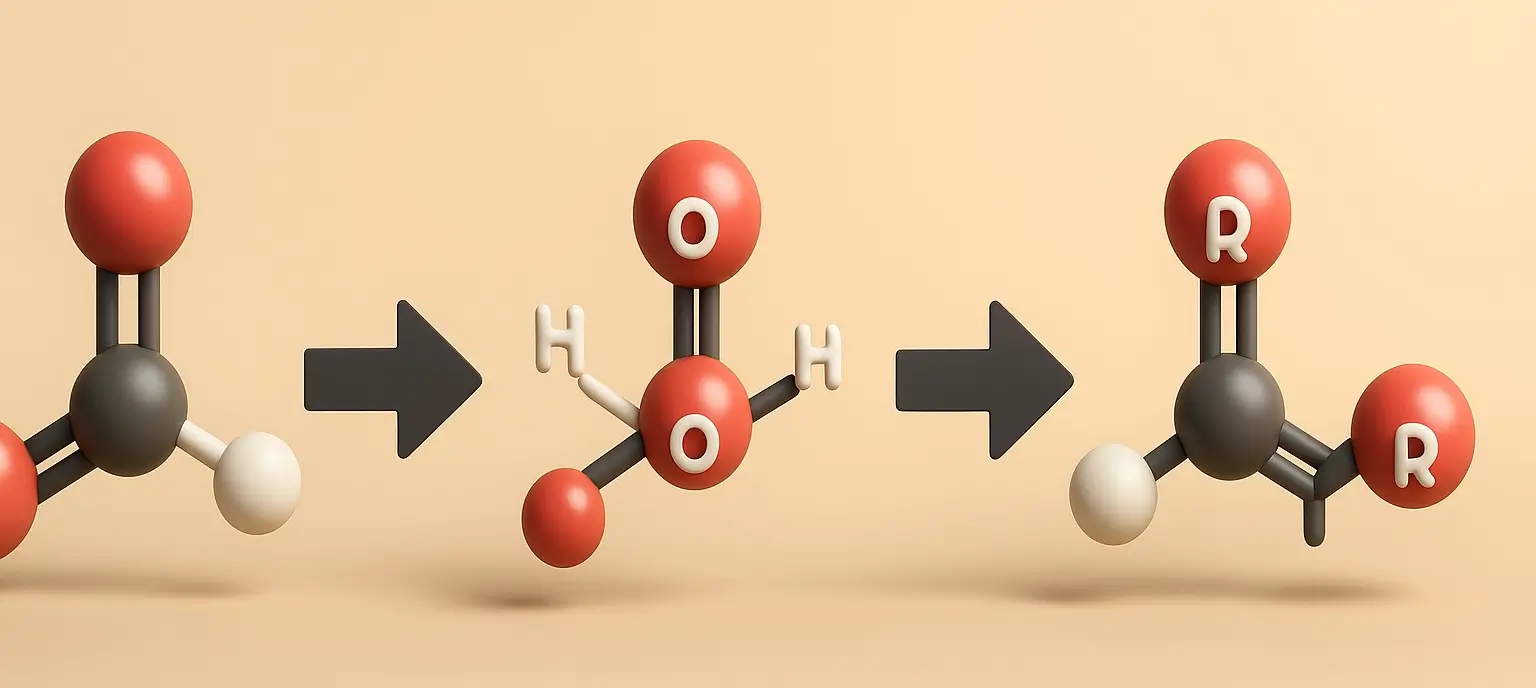
- Carboxylic acid participates in several key chemical reactions due to the reactivity of the carboxyl group.
- Here’s a summary of Carboxylic acid reactions:
Esterification chemical reactions of Carboxylic acid
- Carboxylic acids react with alcohols to form esters and water, typically catalyzed by an acid like sulfuric acid.
- Reaction: RCOOH + R’OH ⟶ RCOOR’ + H₂O
- Example: Acetic Acid and Ethanol to Ethyl Acetate
- CH₃COOH + CH₃CH₂OH ⟶ CH₃COOCH₂CH₃ + H₂O
Acid-Base Reactions
- Carboxylic acids neutralize bases to produce carboxylate salts and water.
- Reaction: RCOOH + MOH ⟶ RCOOM + H₂O
- Example: Acetic Acid and Sodium Hydroxide to Sodium Acetate
- CH₃COOH + NaOH ⟶ CH₃COONa + H₂O
Reduction to Alcohols
- Carboxylic acids can be reduced to primary alcohols using reducing agents such as lithium aluminum hydride (LiAlH₄).
- Reaction: RCOOH + 4[H] ⟶ RCH₂OH
- Example: Acetic Acid to Ethanol
- CH₃COOH + 4[H] ⟶ CH₃CH₂OH
Decarboxylation
- Heating carboxylic acids with a strong base results in the loss of carbon dioxide and formation of an alkane.
- Reaction: RCOOH ⟶ RH + CO₂
- Example: Propanoic Acid to Ethane
- CH₃CH₂COOH ⟶ CH₃CH₃ + CO₂
Formation of Acyl Halides
- Carboxylic acids are converted to acyl halides using halogenating agents like thionyl chloride (SOCl₂).
- Reaction: RCOOH + SOCl₂ ⟶ RCOCl + SO₂ + HCl
- Example: Acetic Acid to Acetyl Chloride
- CH₃COOH + SOCl₂ ⟶ CH₃COCl + SO₂ + HCl
Formation of Amides
- Carboxylic acids react with ammonia or amines to form amides, usually requiring heat or a dehydration agent.
- Reaction: RCOOH + NH₃ ⟶ RCONH₂ + H₂O
- Example: Acetic Acid to Acetamide
- CH₃COOH + NH₃ ⟶ CH₃CONH₂ + H₂O
These reactions highlight the diverse reactivity of carboxylic acids, making them key intermediates in various chemical syntheses.
Click Here to Watch the Best Pharma Videos