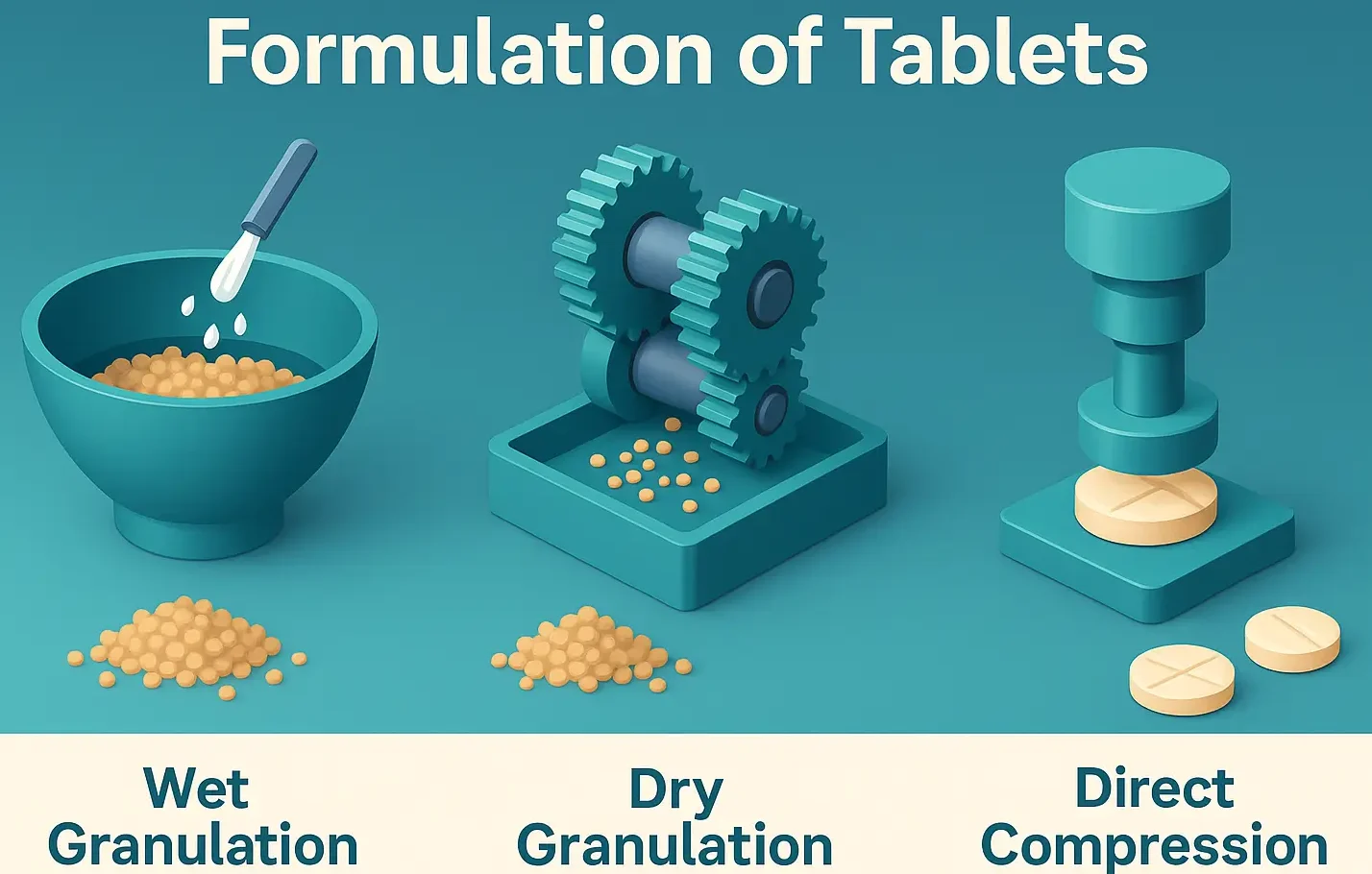
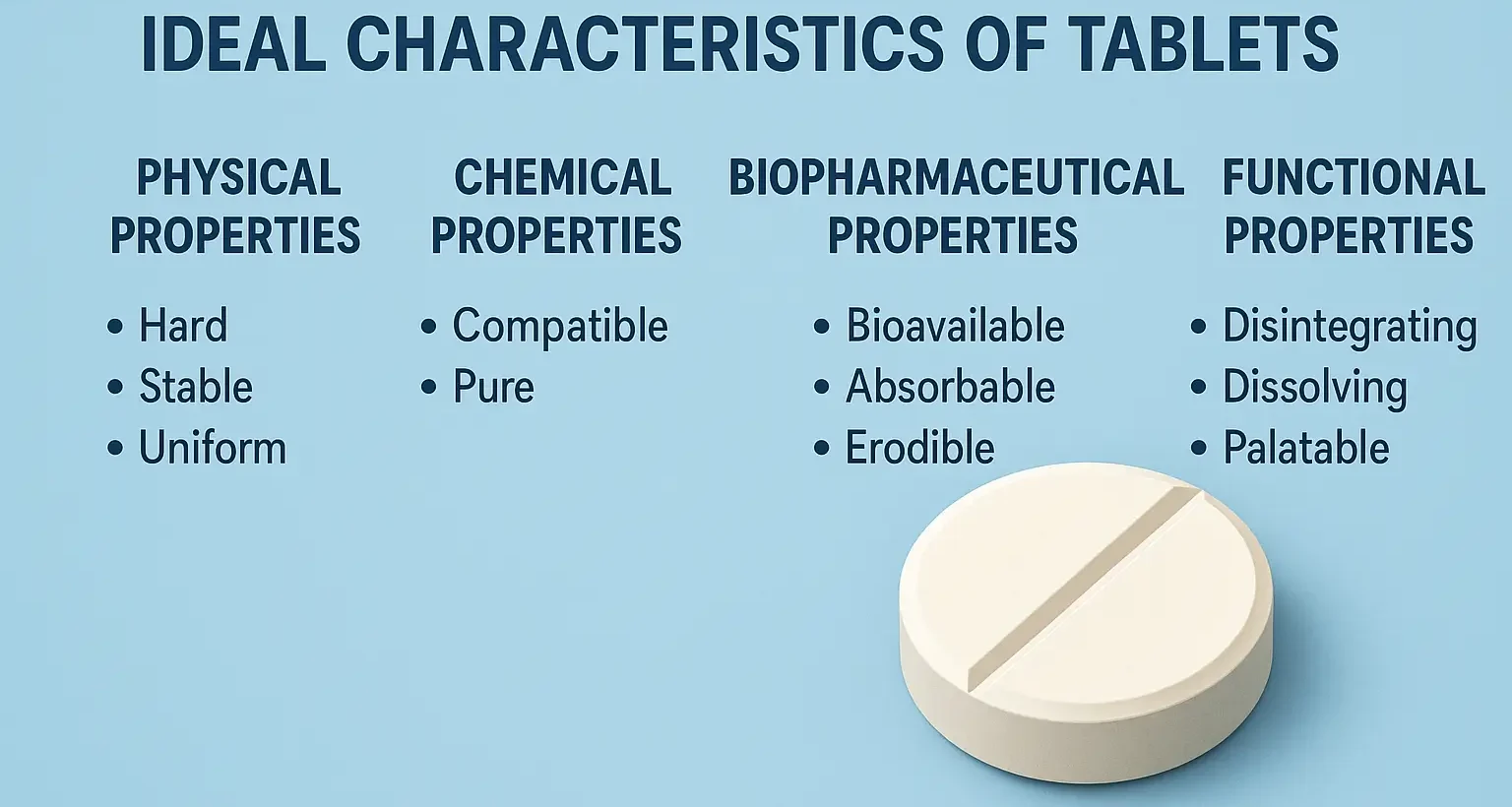
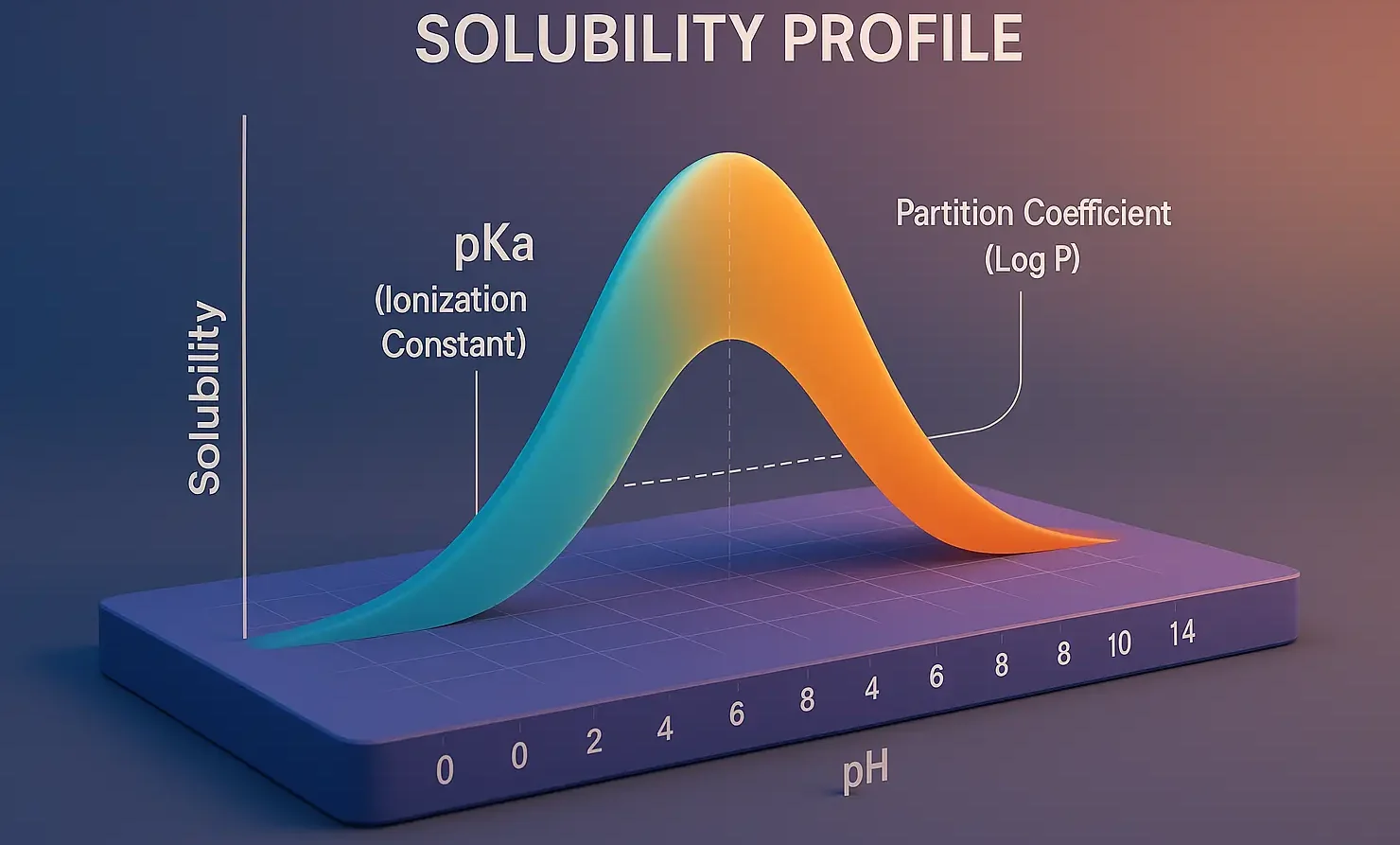
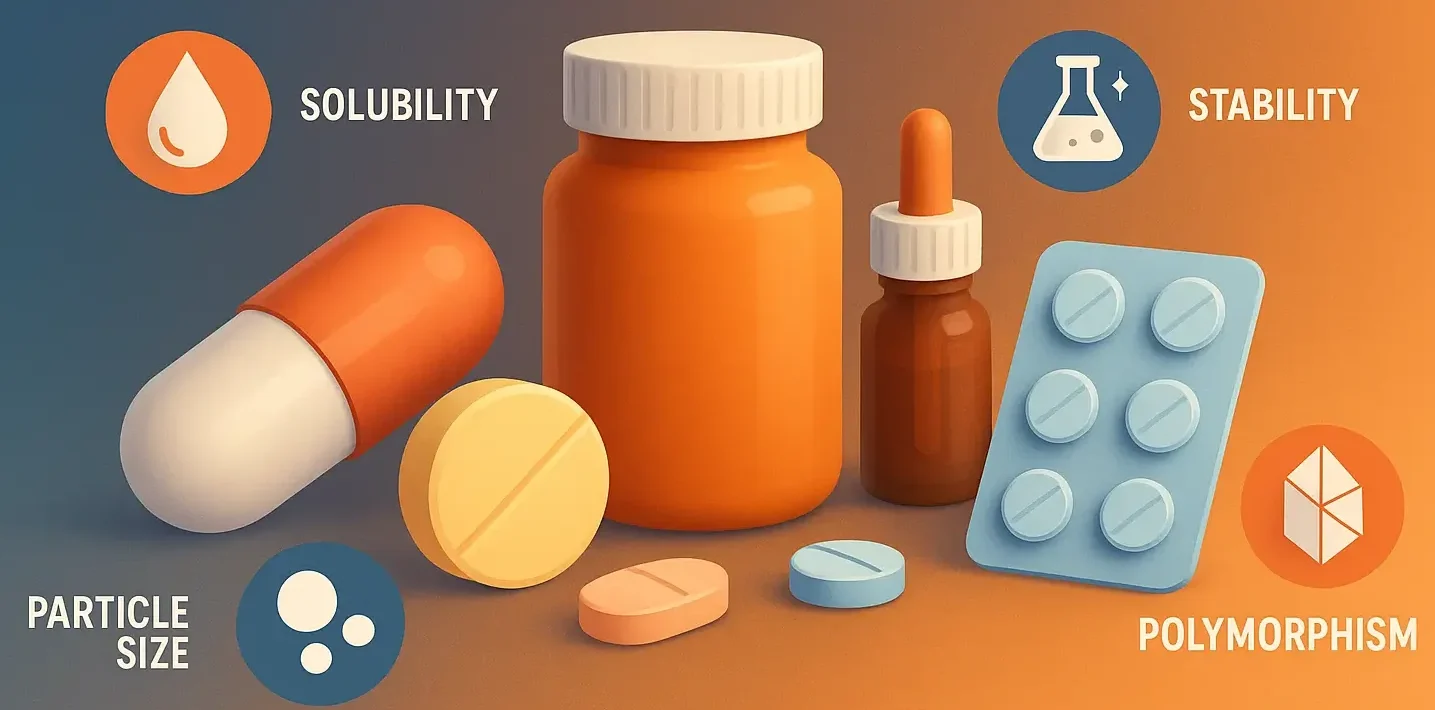
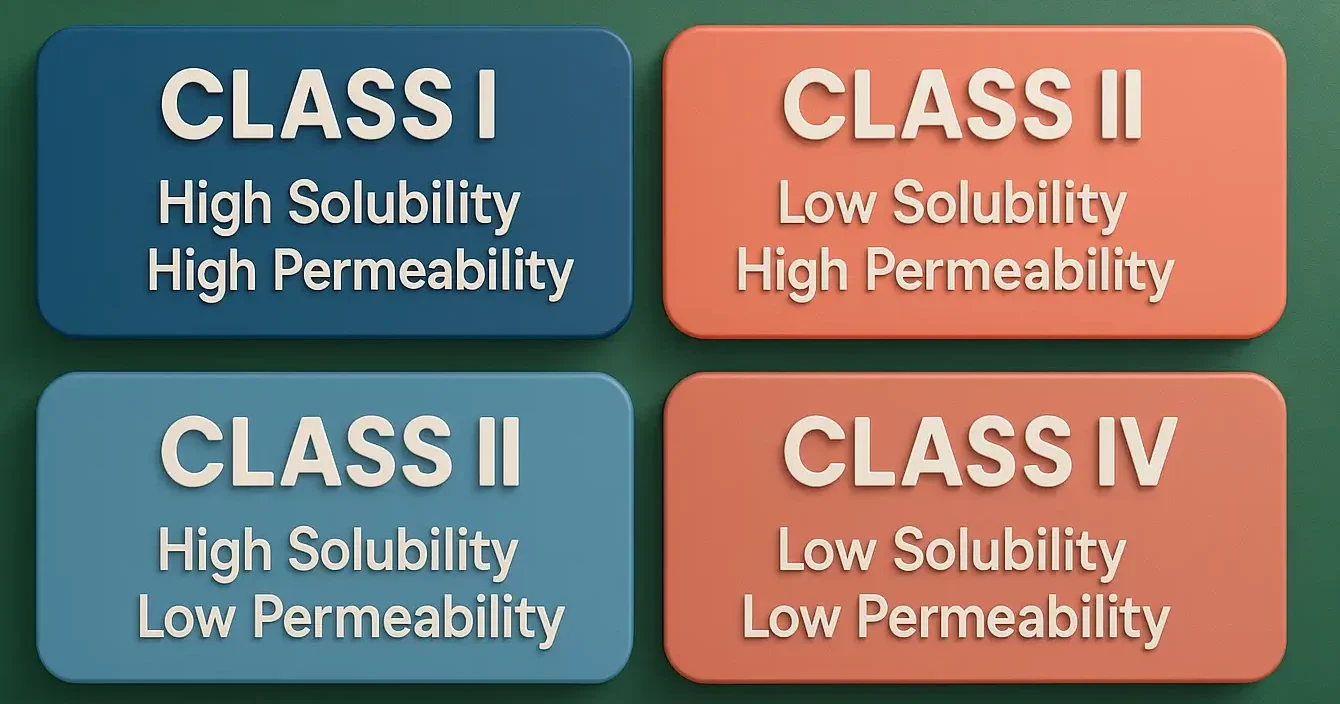
Formulation of Tablets
Formulation of Tablets involves selecting suitable excipients and processing methods to create effective, stable dosage forms. It includes drug blending, granulation, compression, and coating for consistent drug release and patient compliance. Formulating a tablet involves selecting appropriate APIs and excipients and determining their proportions to achieve desired tablet properties. The formulation process includes: Steps involved … Read more