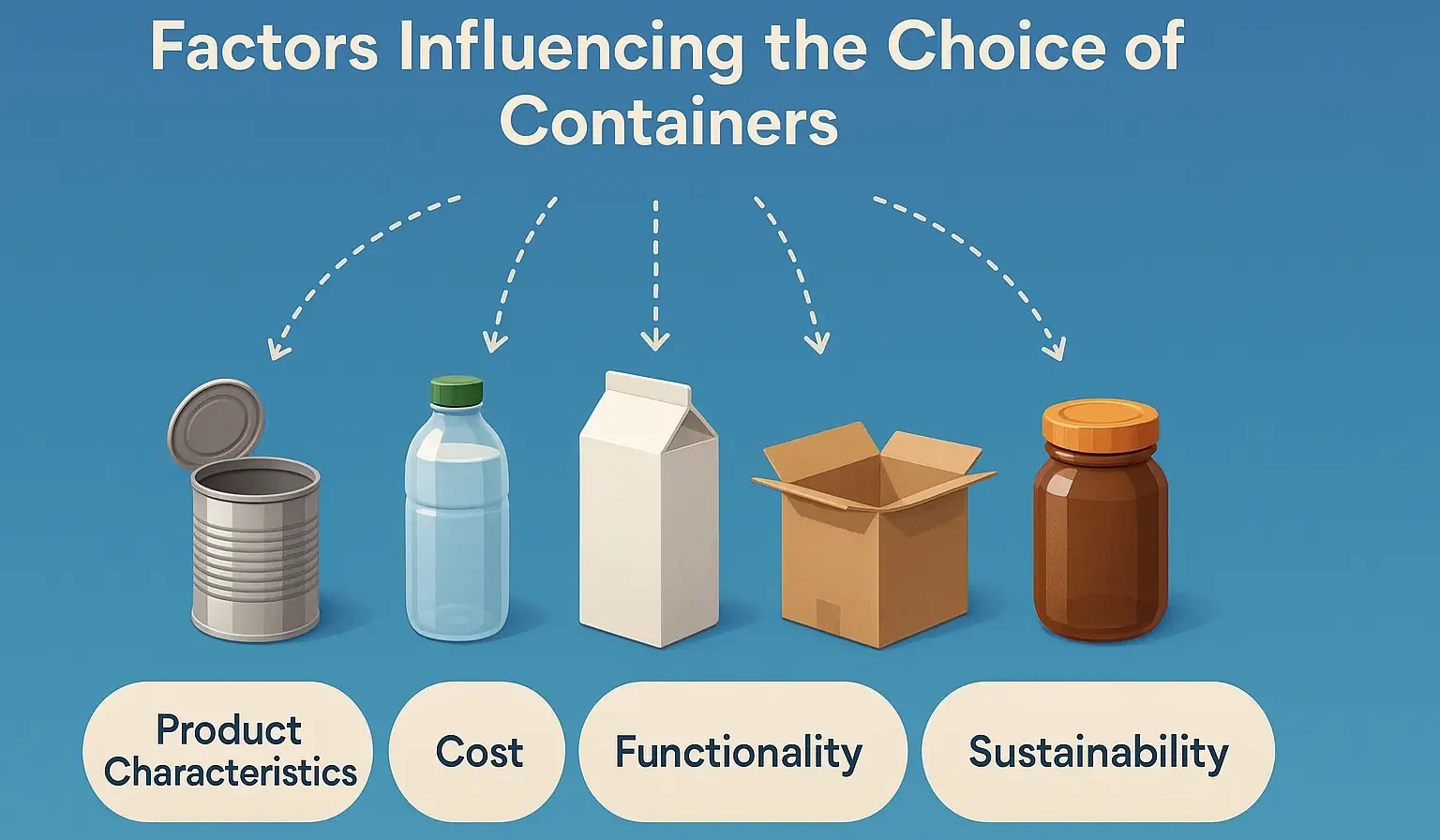
Quality Control Tests for Packaging Materials
Quality Control Tests for Packaging Materials ensure strength, integrity, and compatibility by assessing parameters like tensile strength, thickness, and permeability. Quality Control Tests for Packaging Materials also check microbial contamination, chemical reactivity, and environmental stability to maintain product safety and compliance. Robust quality control (QC) testing is essential to ensure that packaging components meet the … Read more