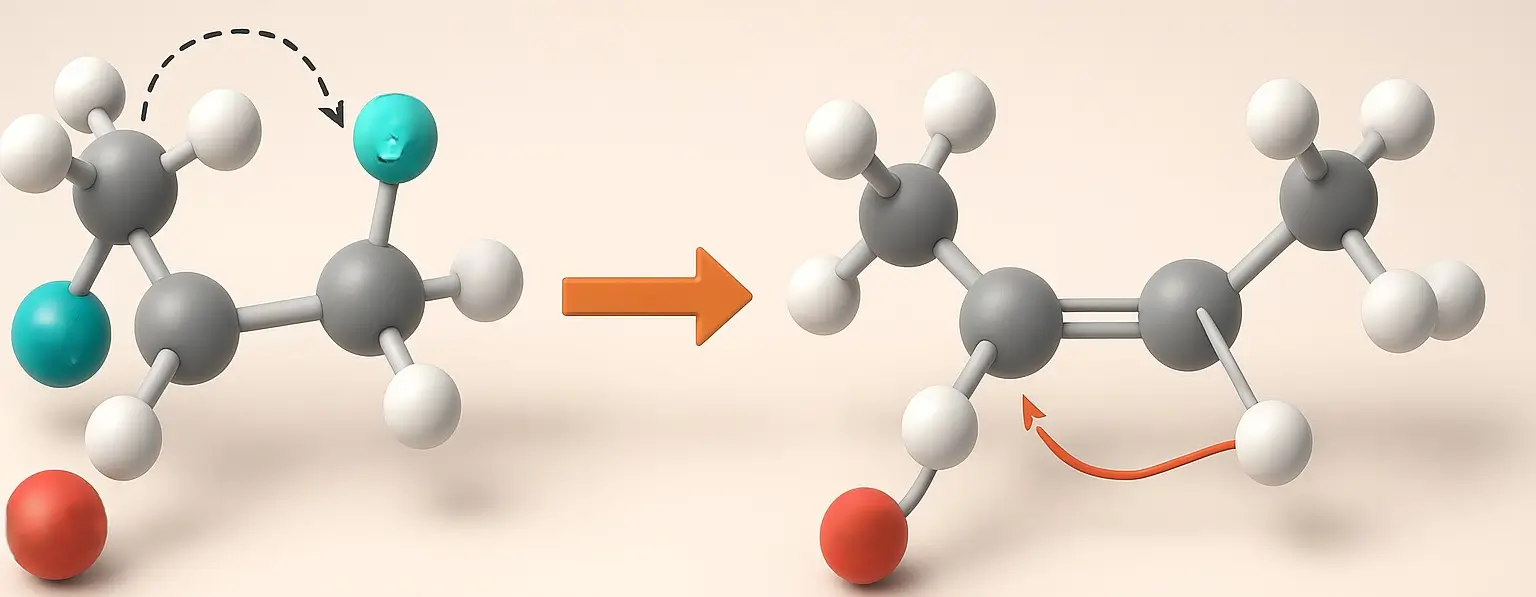
Diels-alder reaction
Diels-Alder reaction is a [4+2] cycloaddition reaction between a conjugated diene and a dienophile (an alkene or alkyne) to form a six-membered cyclic compound. It proceeds via a concerted mechanism, meaning the bonds are formed simultaneously without intermediates. This reaction is stereospecific and regioselective, typically occurring under mild conditions and often accelerated by heat or … Read more