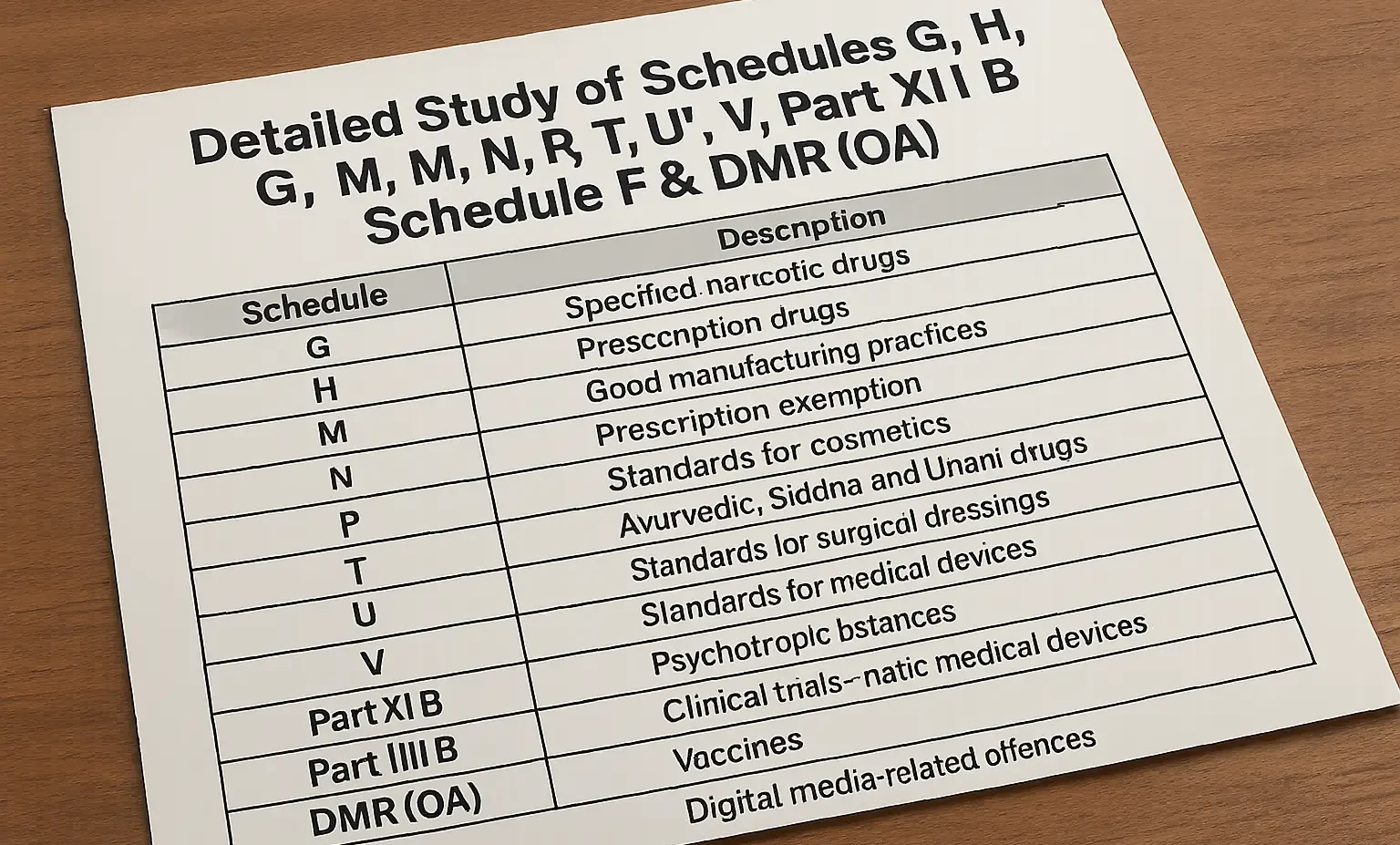
Detailed Study of Schedules G, H, M, N, P, T, U, V, X, Y, Part XII B, Schedule F & DMR (OA)
Explore the study of important drug schedules and DMR OA under Indian regulations with their legal relevance and compliance requirements. The Schedules in the Drugs and Cosmetics Act classify drugs based on various criteria, including their potential for abuse, control measures, and prescribing regulations. Detailed Study of Important Drug Schedules and DMR OA, Parts in … Read more