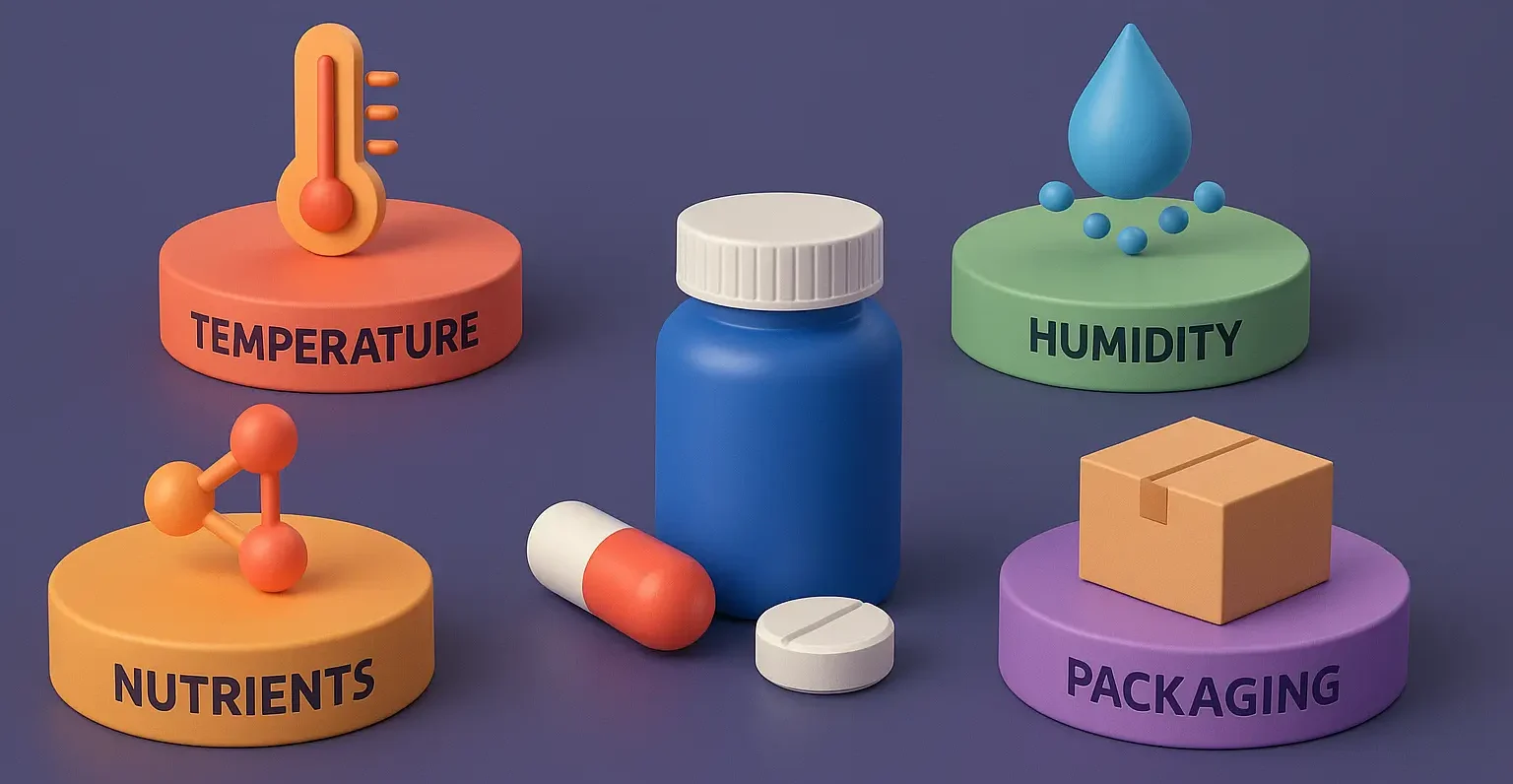
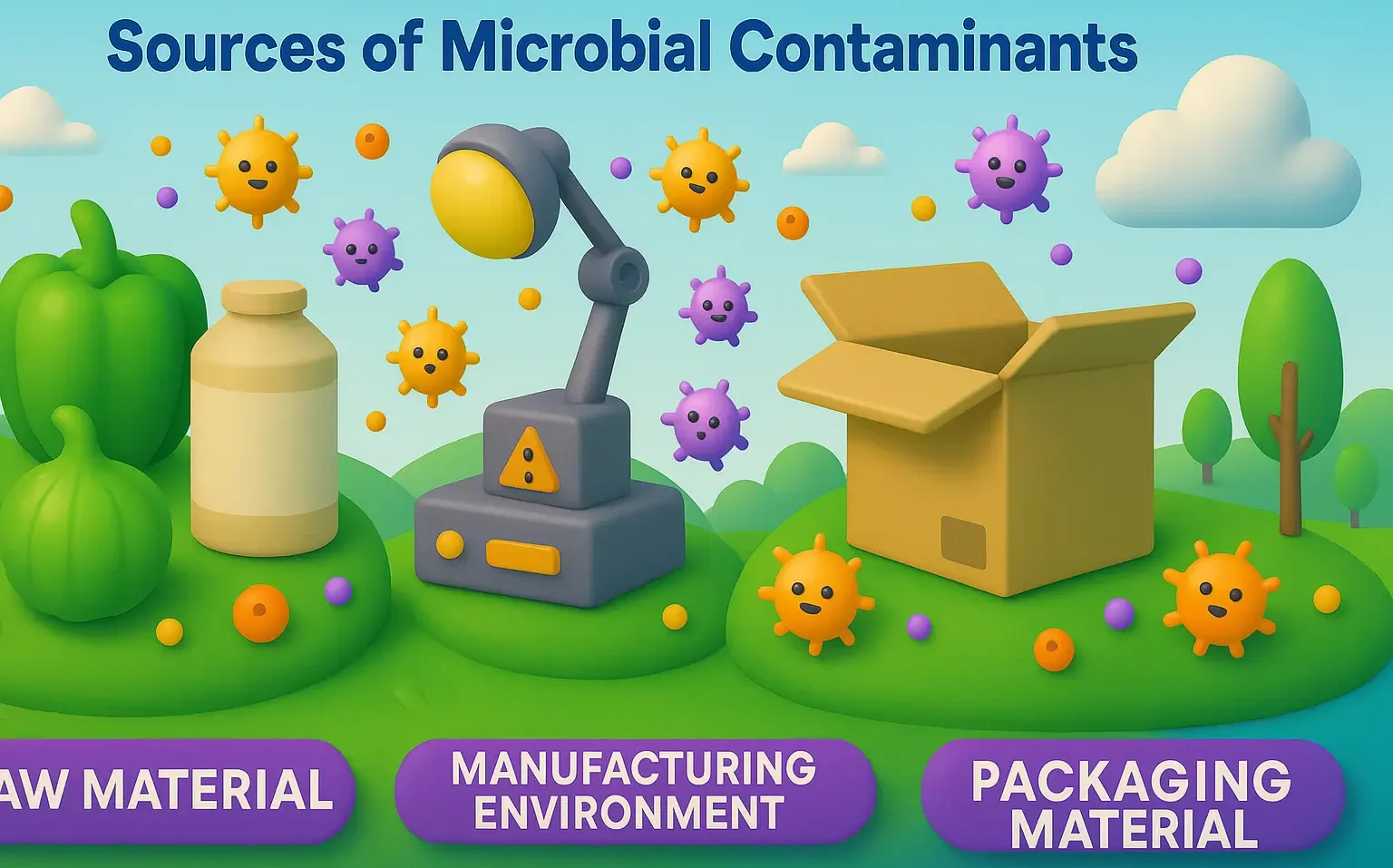
Application of Cell Cultures in Pharmaceutical Industry and Research
Application of Cell cultures are used in pharma for drug development, toxicity testing, vaccine production, and disease modeling, enabling safer, faster, and cost-effective research and manufacturing processes. Below are some most common Application of Cell Cultures in Pharmaceutical Industry and Research: Drug Discovery and Development: Screening of potential drug candidates for efficacy and cytotoxicity. Studying … Read more