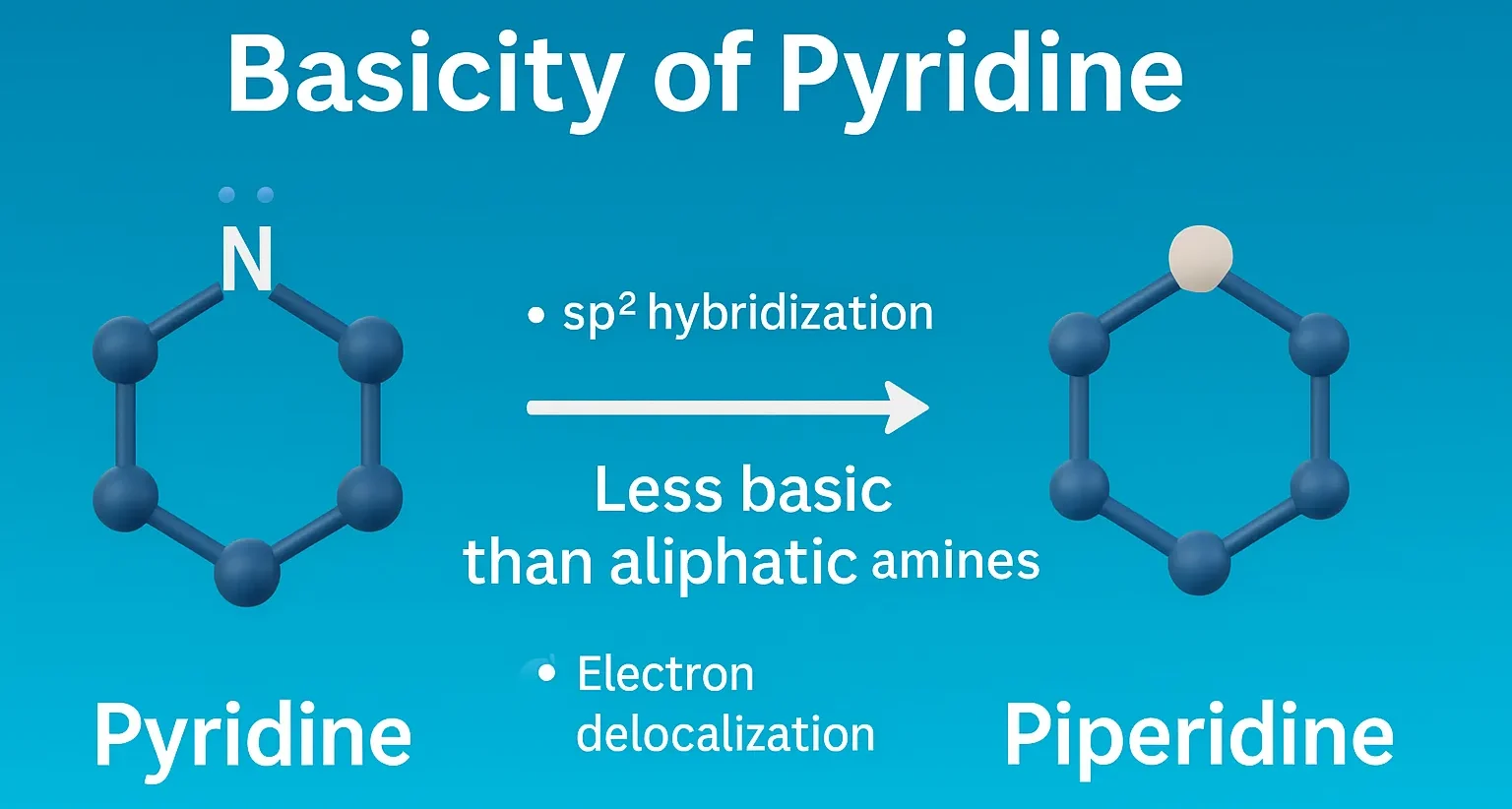
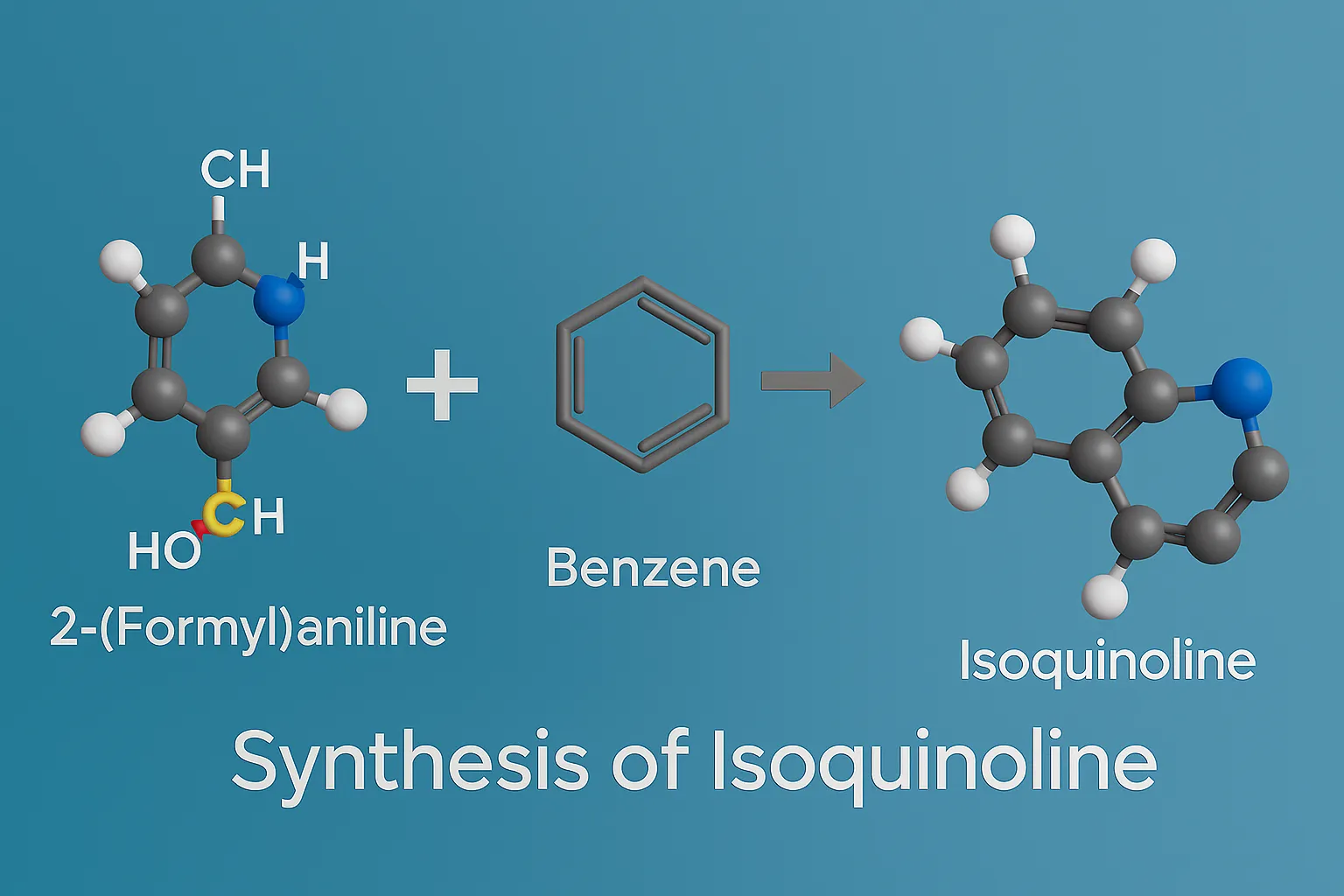
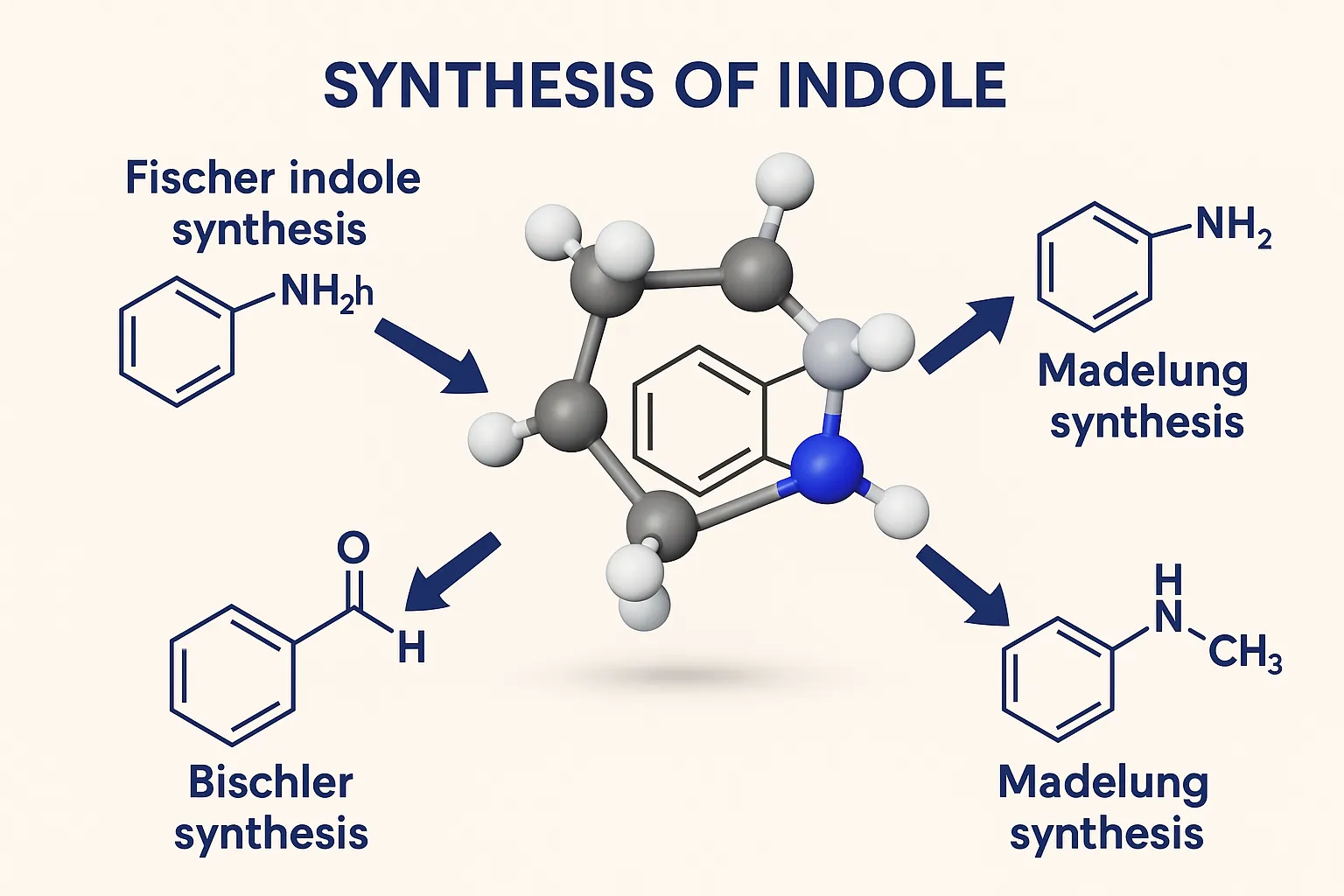
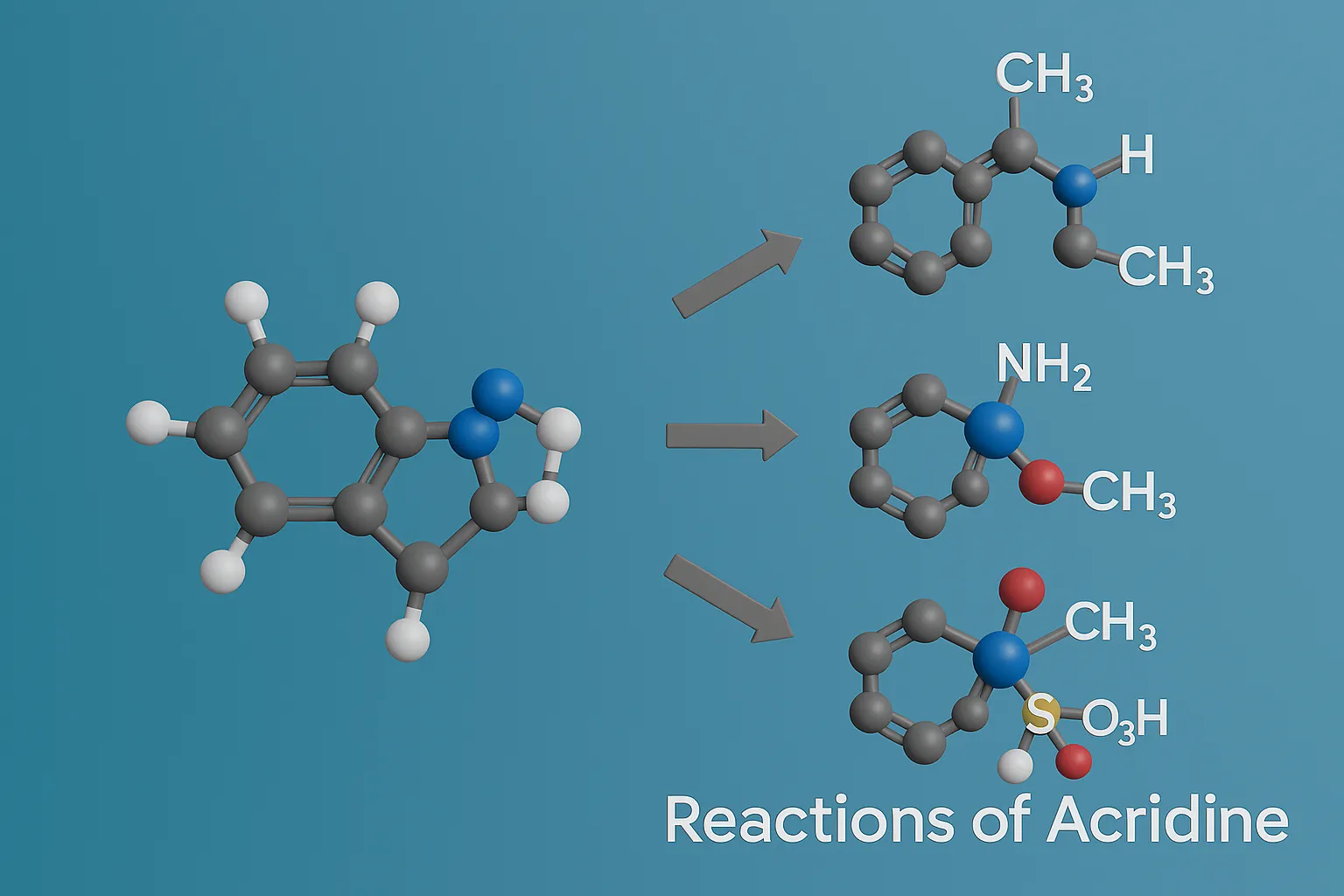
Purine
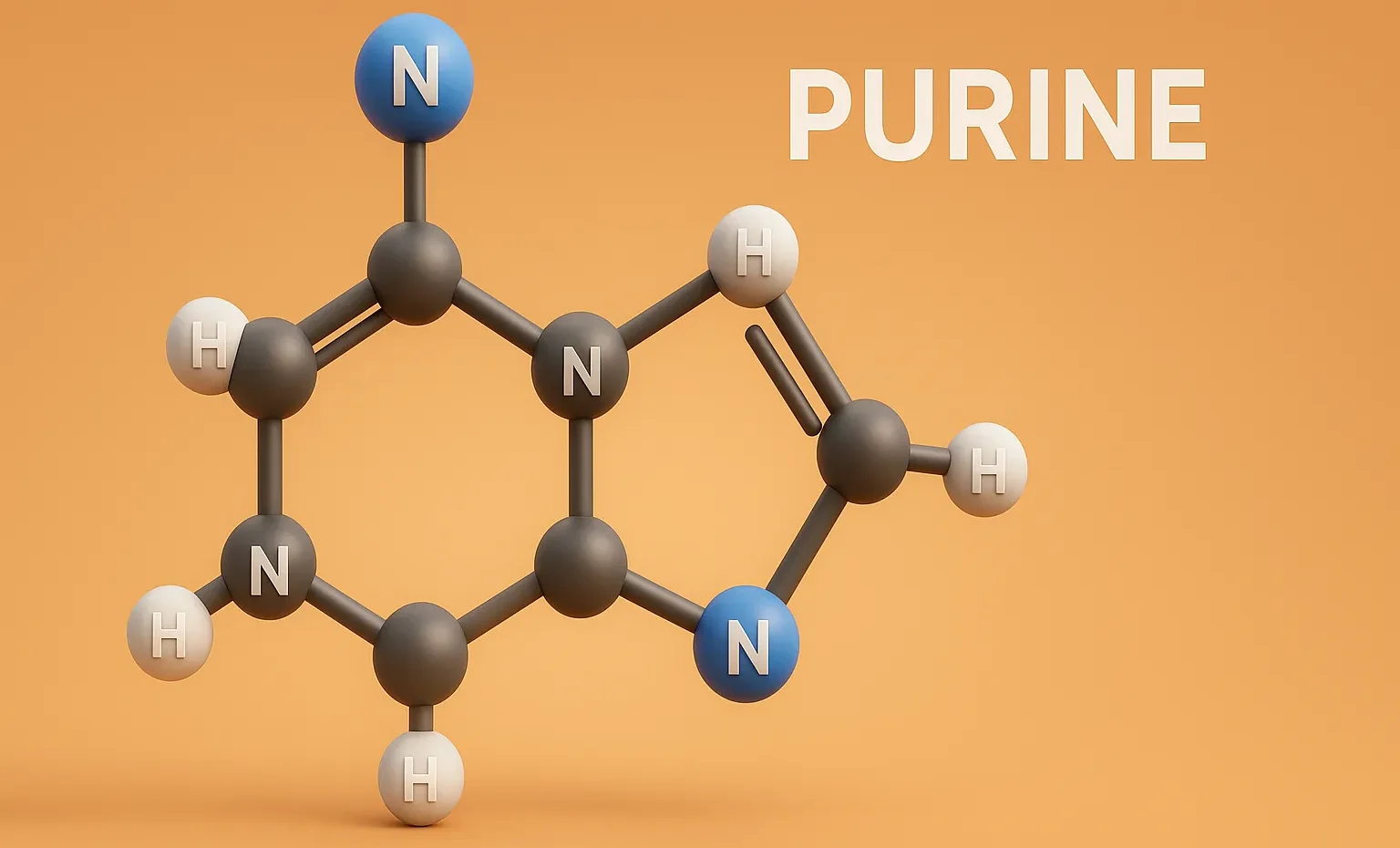
Purine is a bicyclic heterocyclic compound forming the basis of nucleic acids, coenzymes, and many pharmaceutical agents. Structure A fused bicyclic ring: pyrimidine ring fused with imidazole. Molecular formula: C₅H₄N₄ Found in DNA and RNA (adenine and guanine). Synthesis Traube Synthesis Multi-step process starting from 4,5-diaminopyrimidine, formic acid or formamide, then cyclization. Produces purine nucleus. … Read more