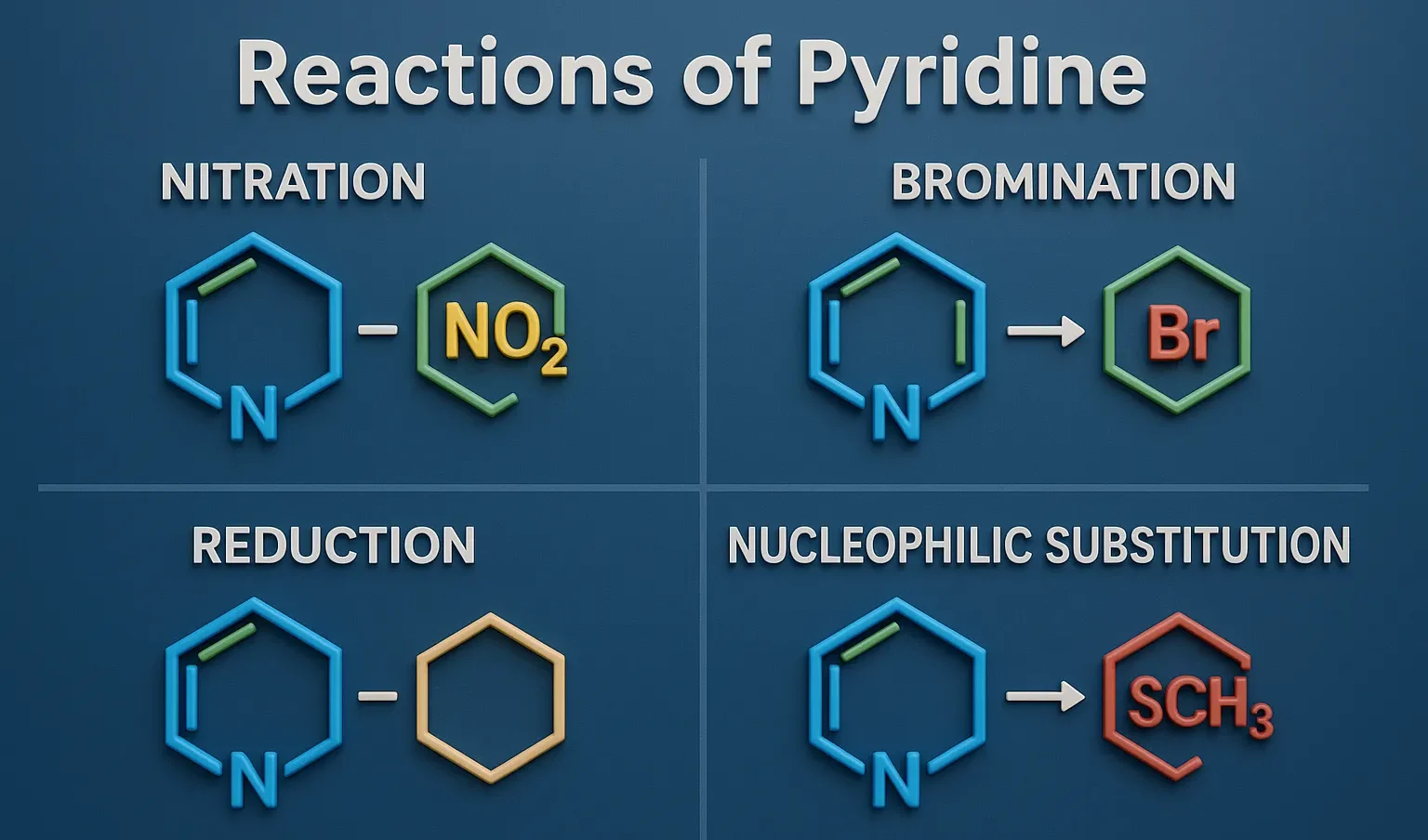
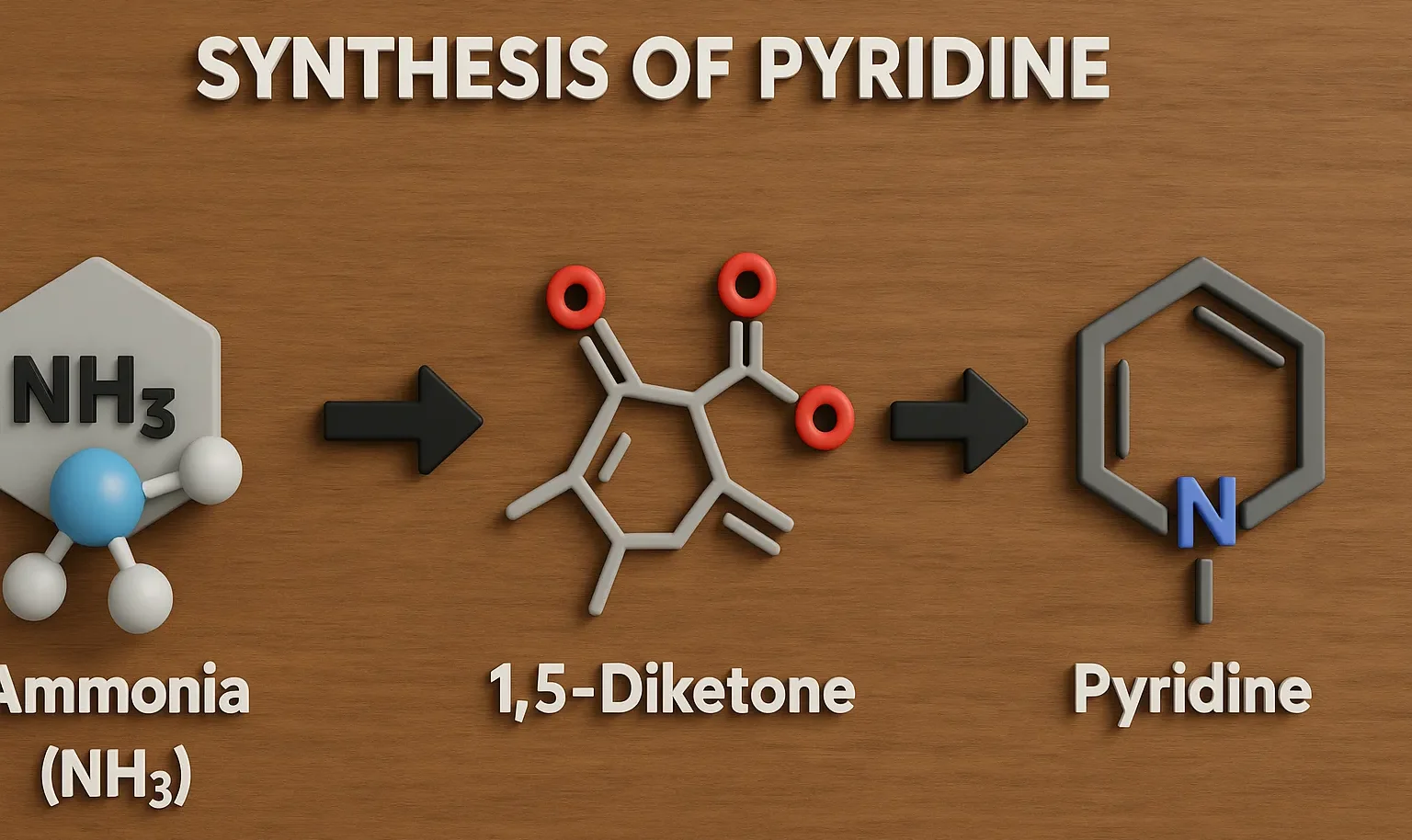
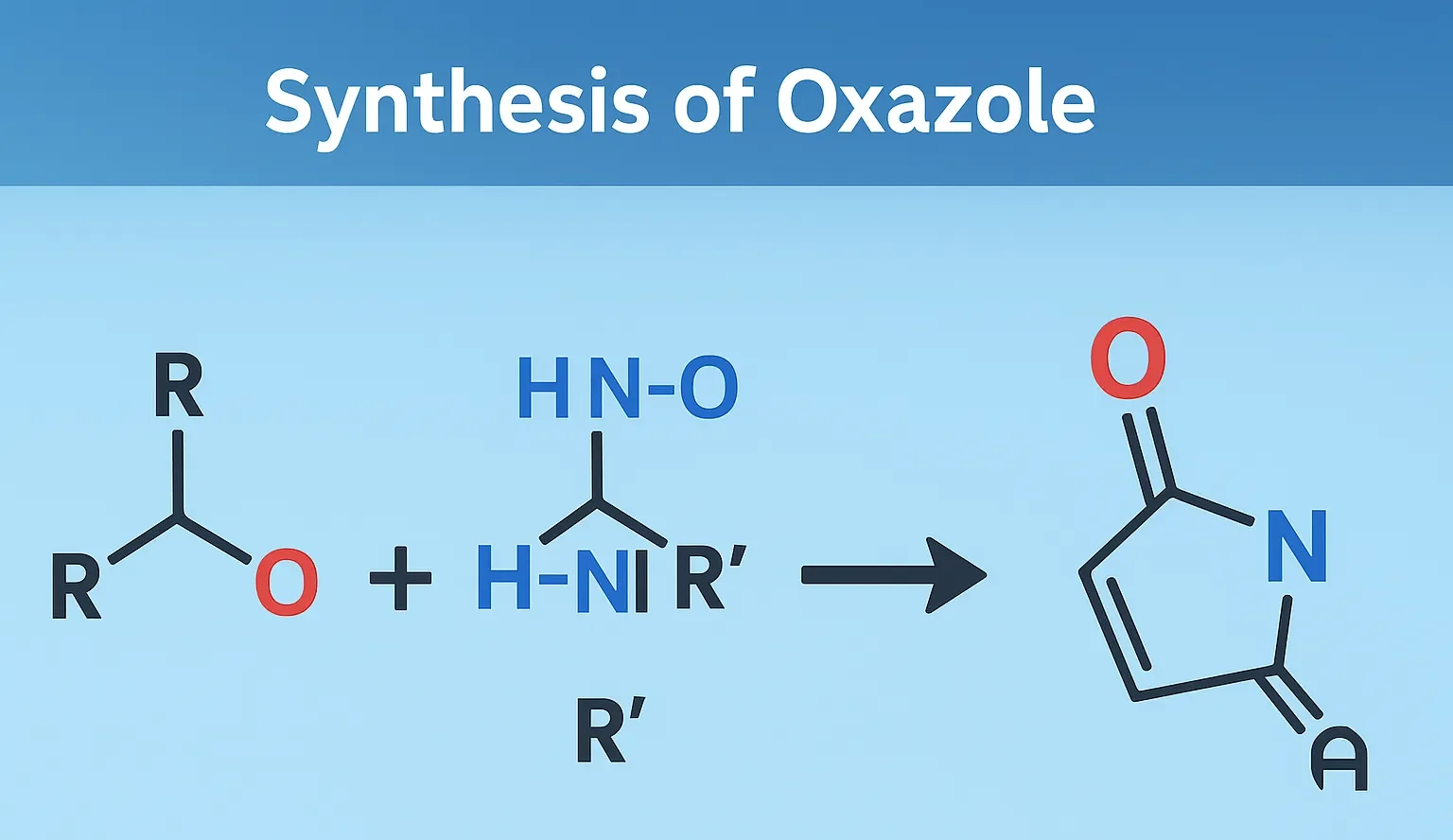
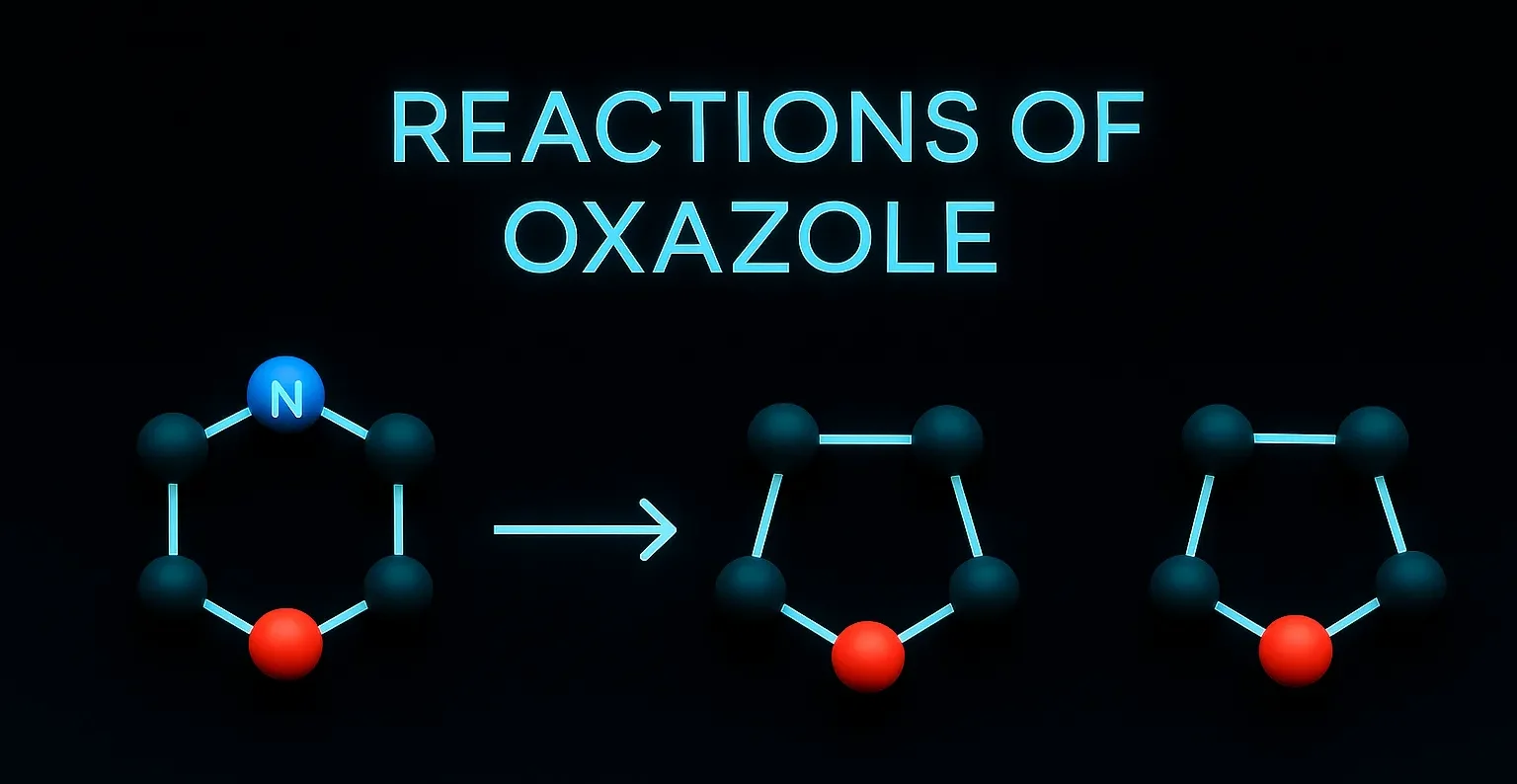
Acridine
Acridine is a nitrogen heterocyclic compound used in dyes, antiseptics, and anticancer research in medicinal chemistry. Chemical Formula of Acridine: C₁₃H₉N Physical Properties of Acridine: Property Value Appearance Yellow crystalline solid Melting Point ~110 °C Boiling Point ~345 °C Solubility Slightly soluble in water, soluble in ethanol and ether Basicity pKa ~5.5 Medicinal Uses: Used … Read more