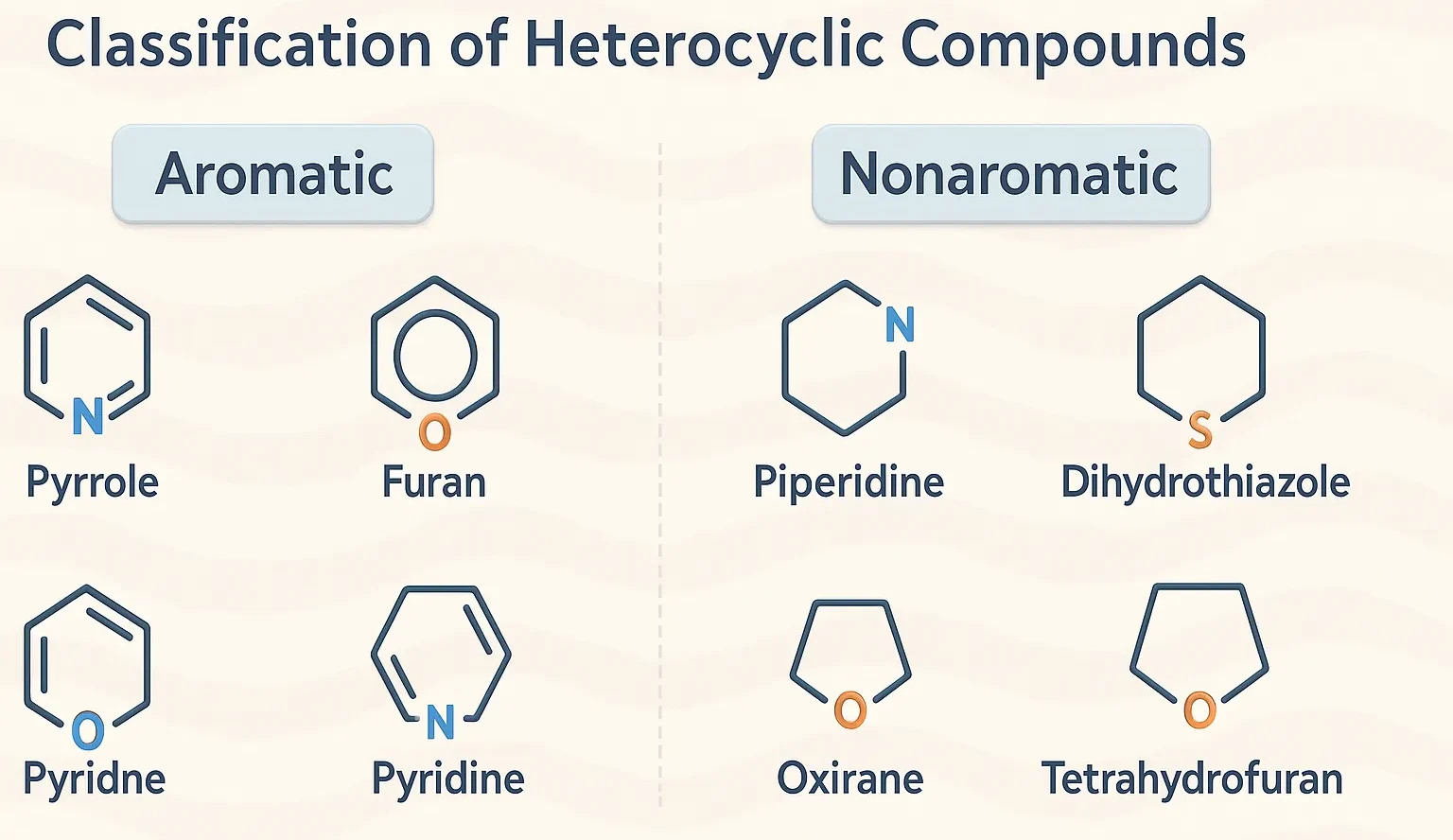
Pyrazole
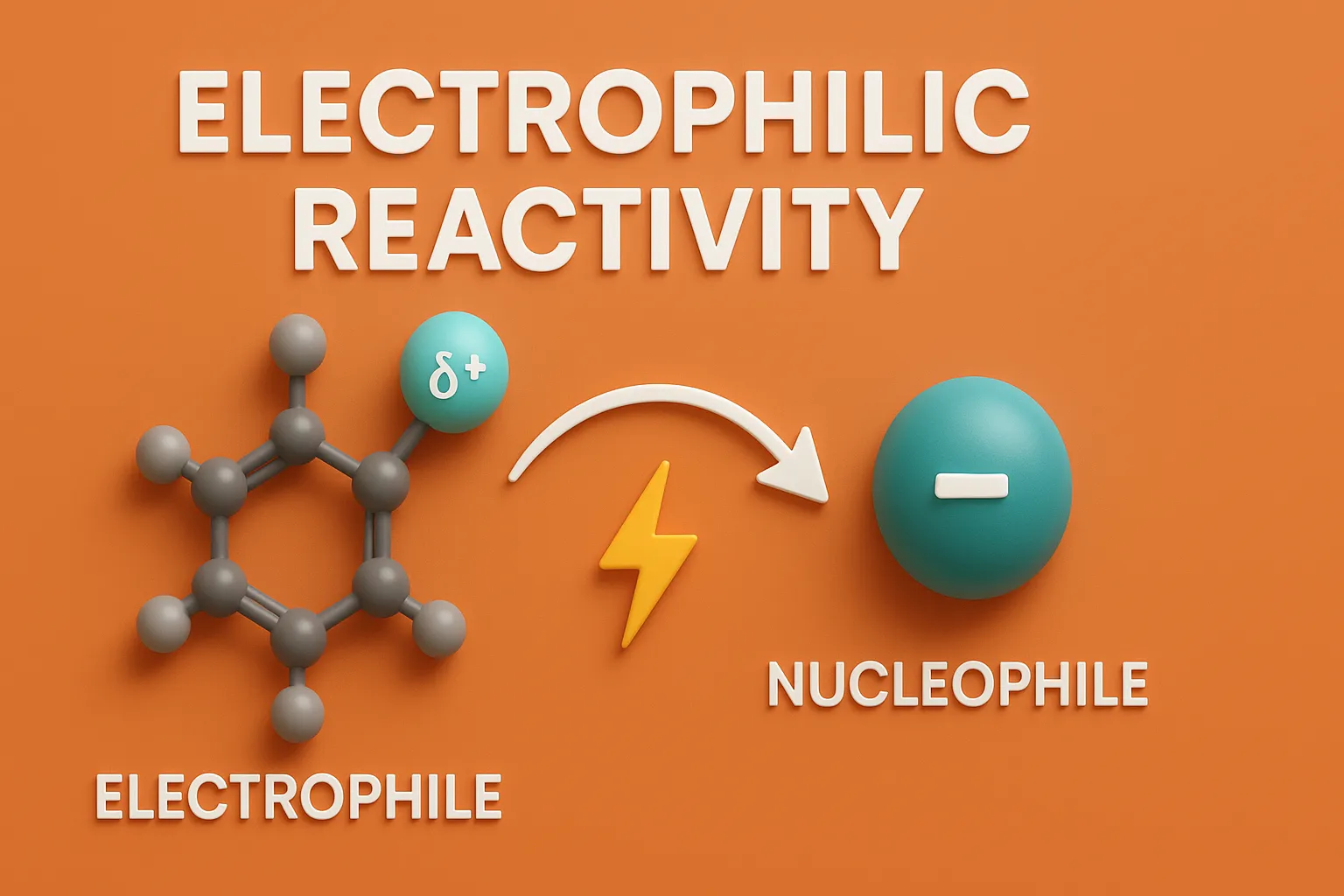
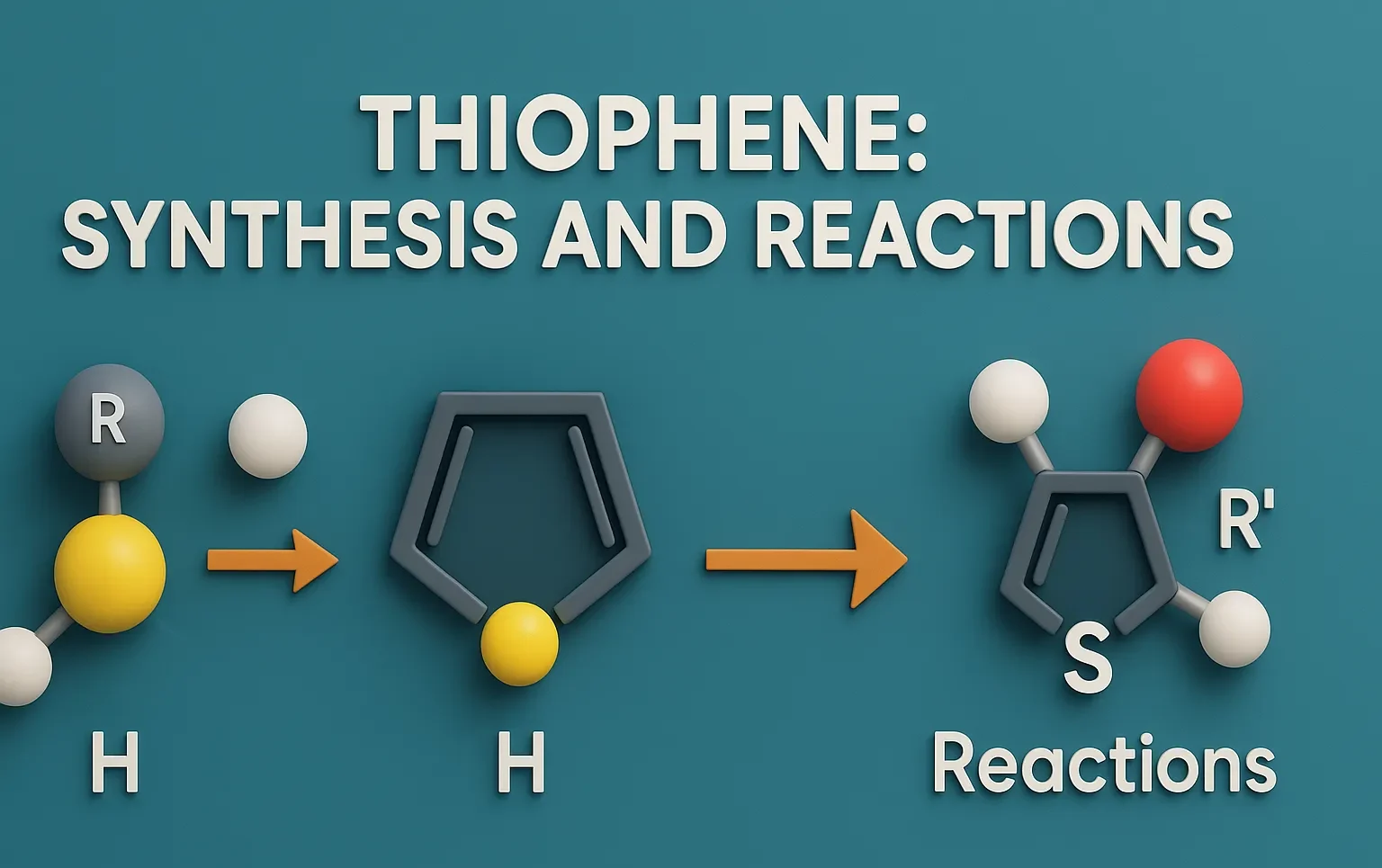
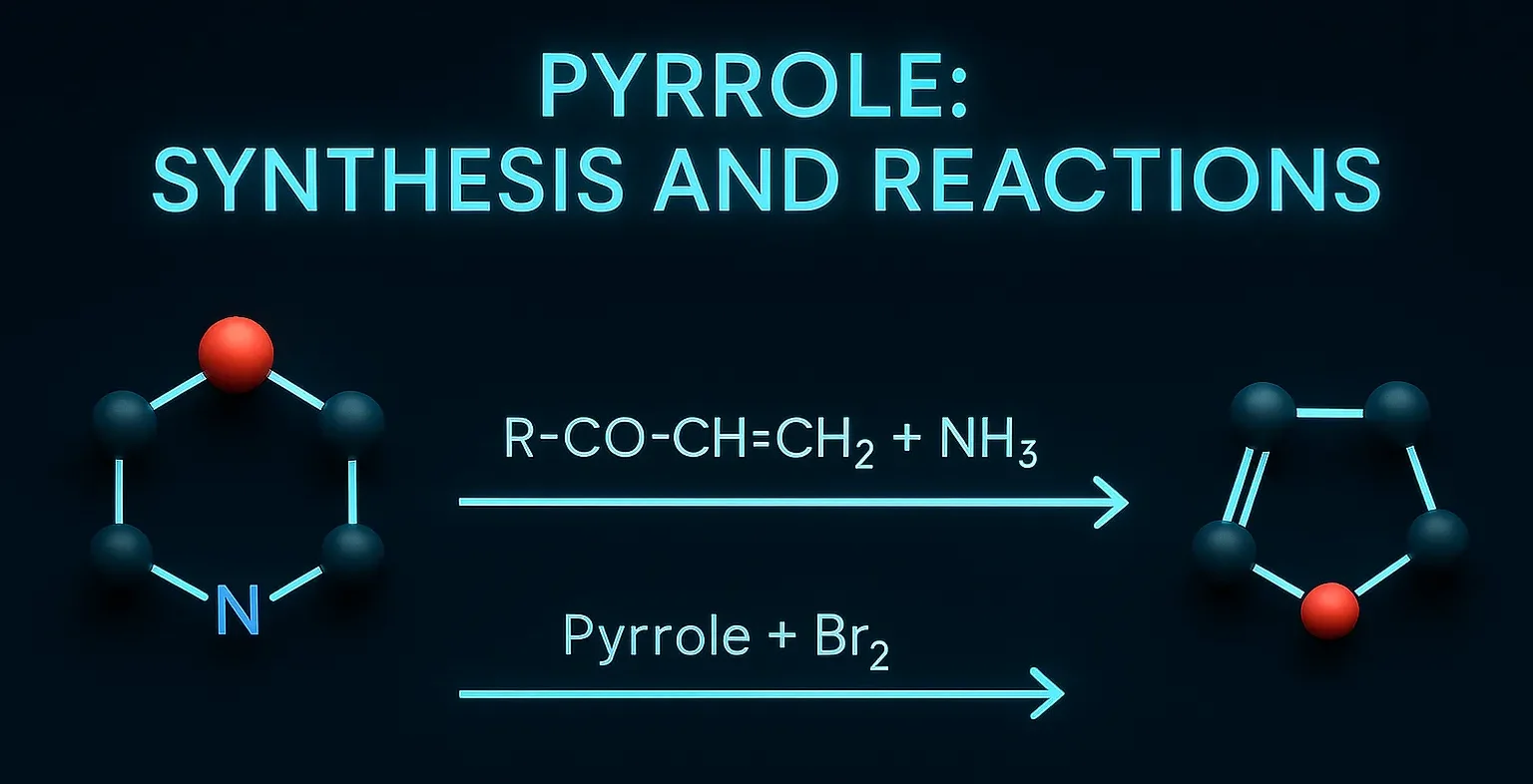
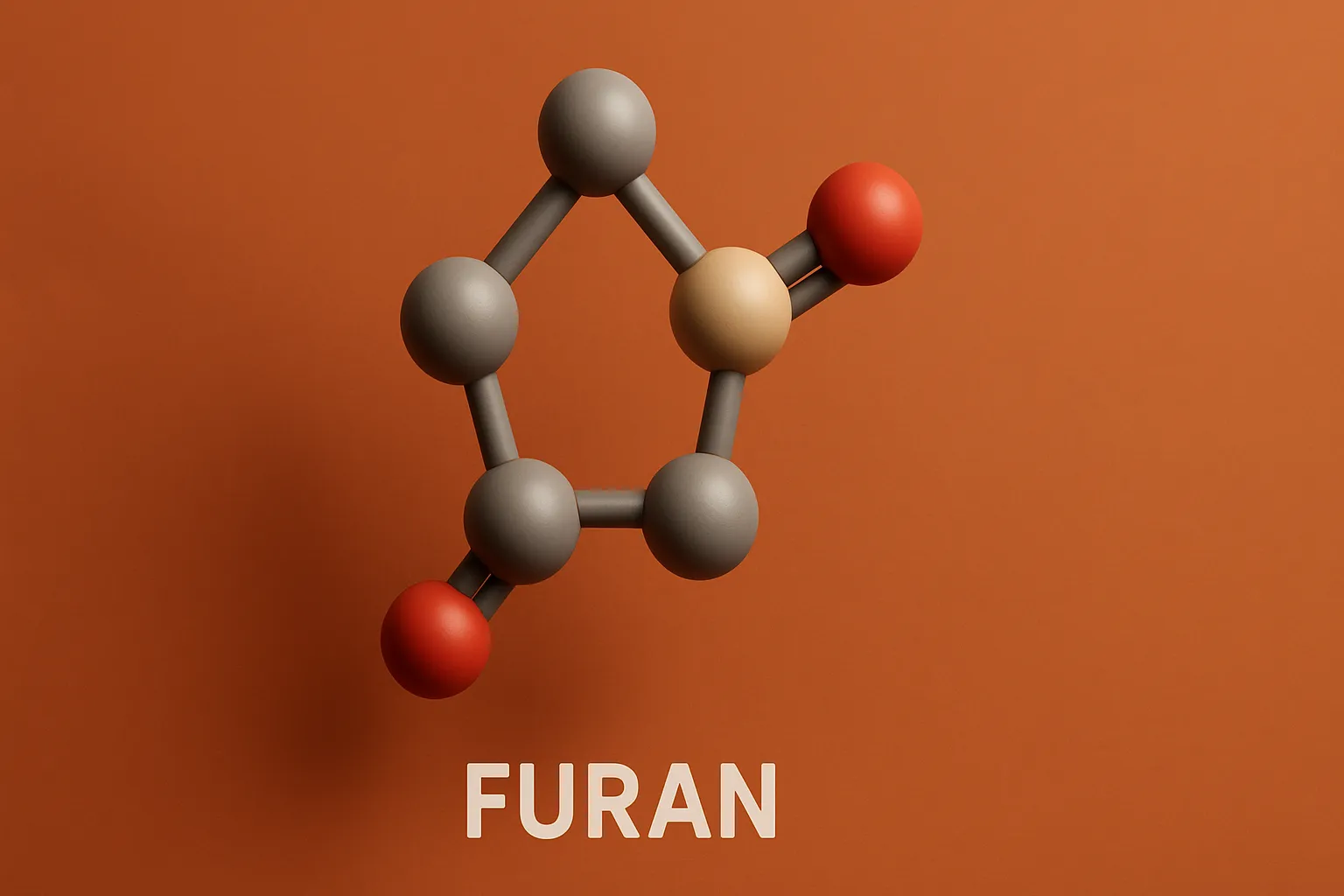
Pyrazole is a five-membered aromatic heterocyclic compound with two adjacent nitrogen atoms, used in medicines and agrochemicals. Chemical Formula of Pyrazole: C₃H₄N₂ Physical Properties: Property Value Appearance White crystalline solid Melting Point ~66 °C Boiling Point ~186 °C Solubility Soluble in water, alcohol, ether Basicity Weakly basic Medicinal Uses: Found in analgesic, anti-inflammatory, antipyretic, and … Read more