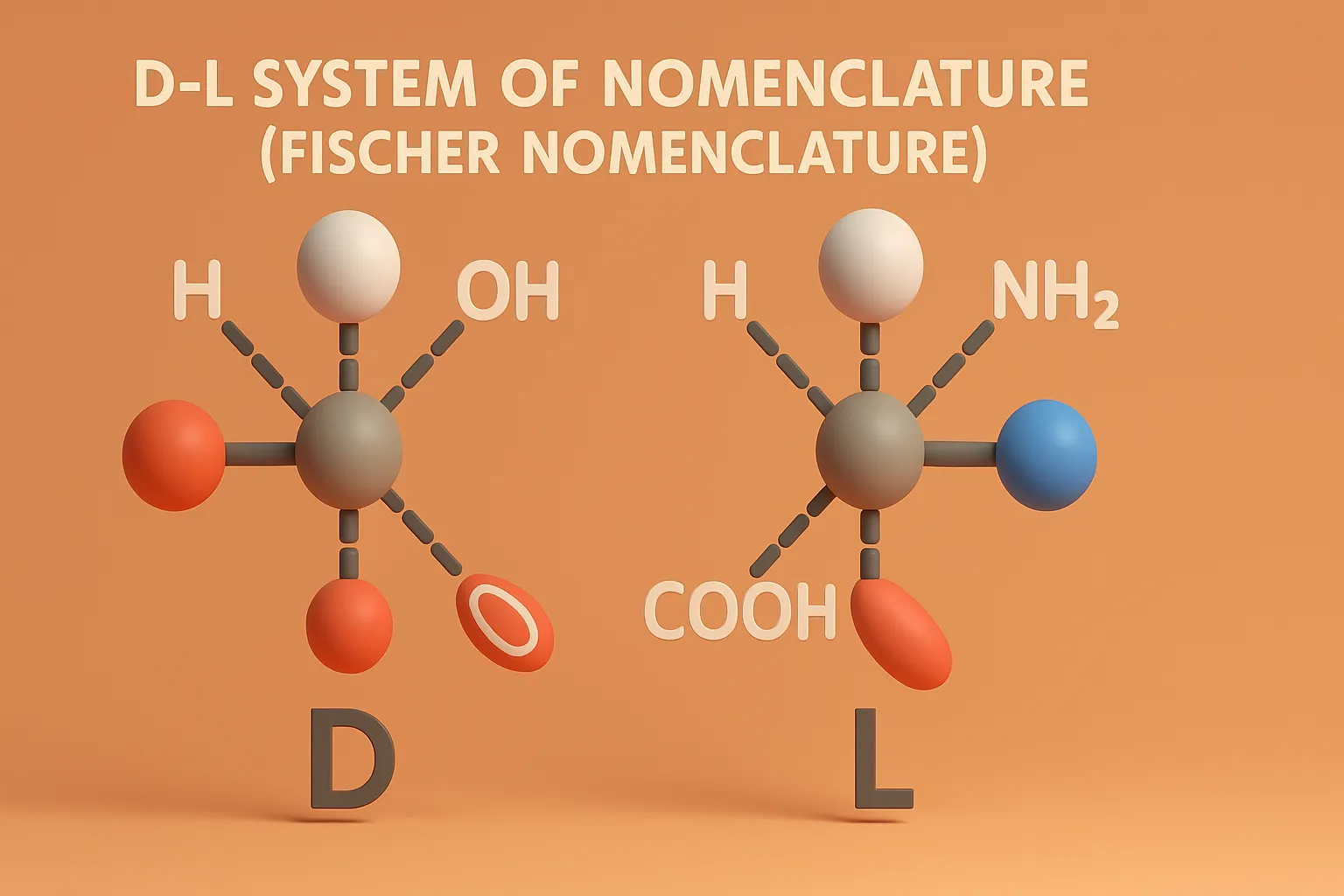
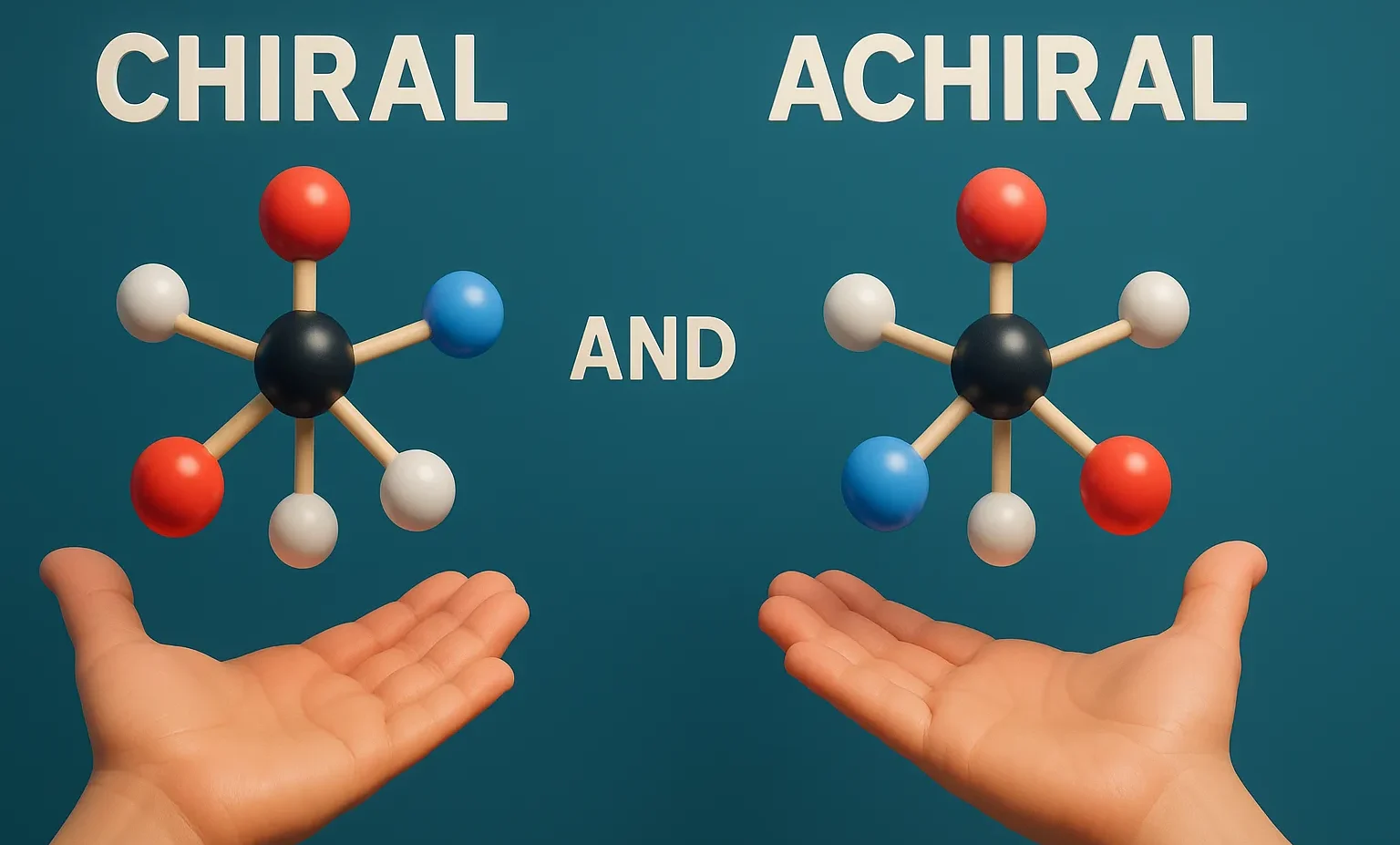
Sequence Rules (Cahn–Ingold–Prelog Rules)
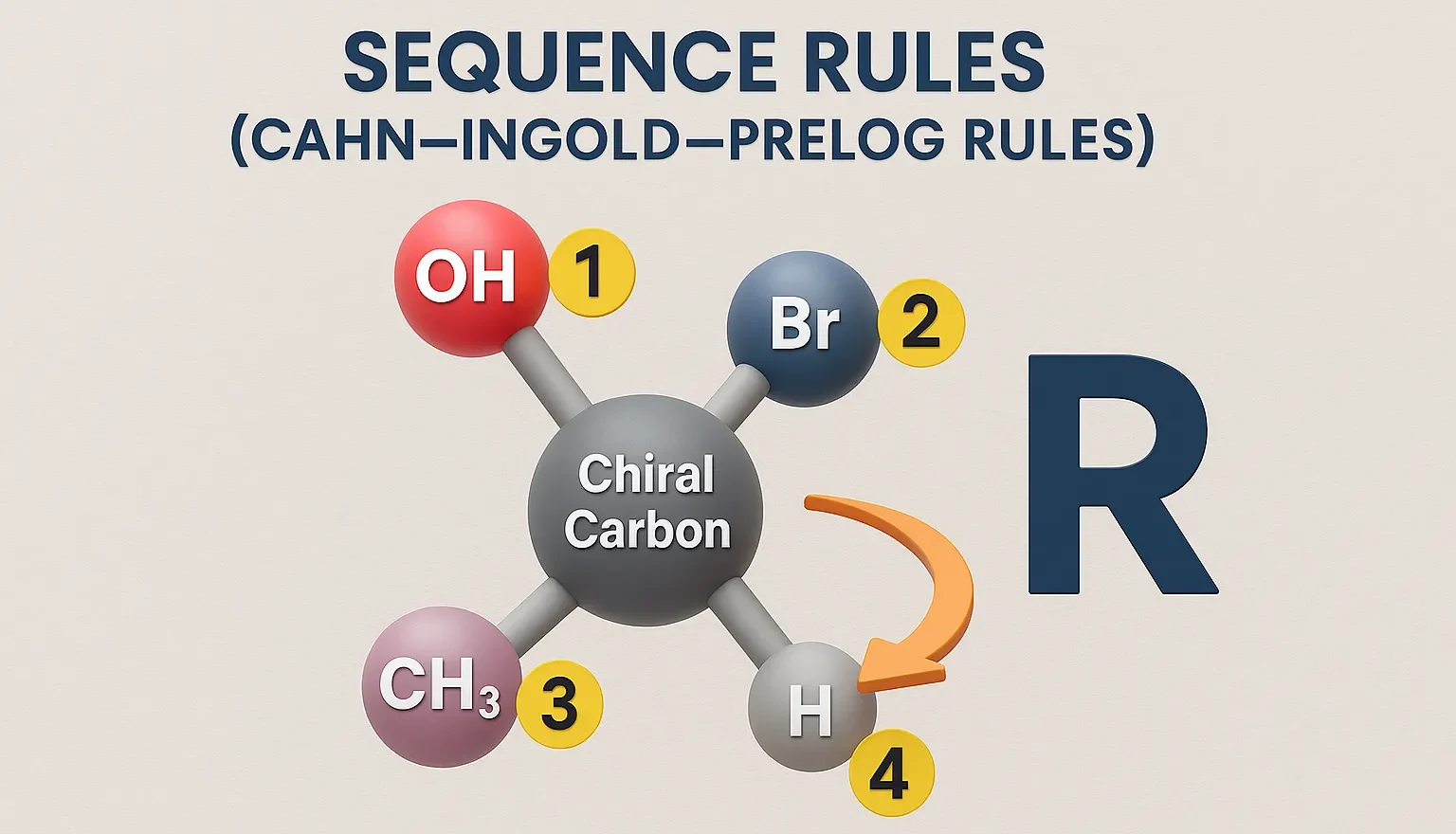
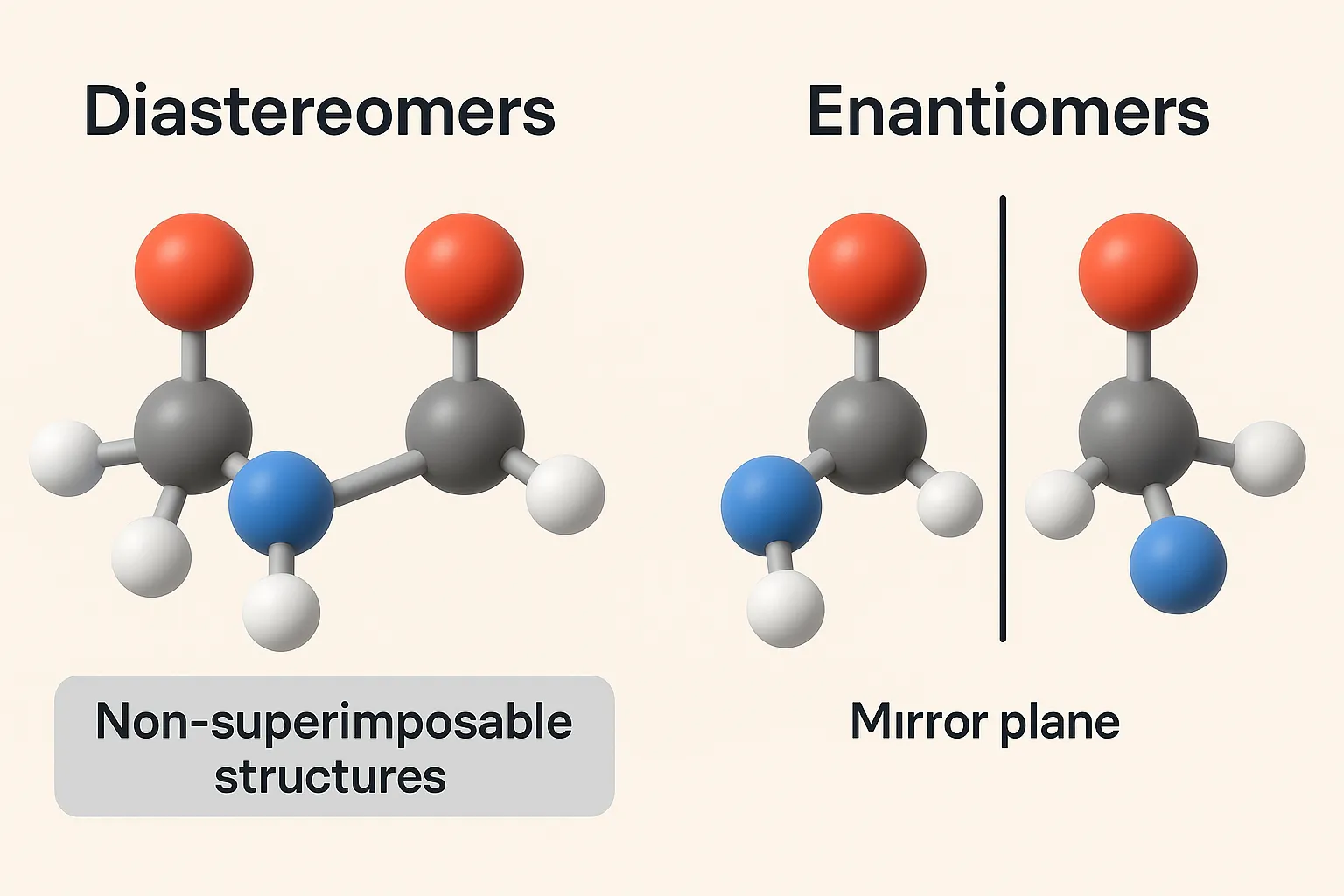
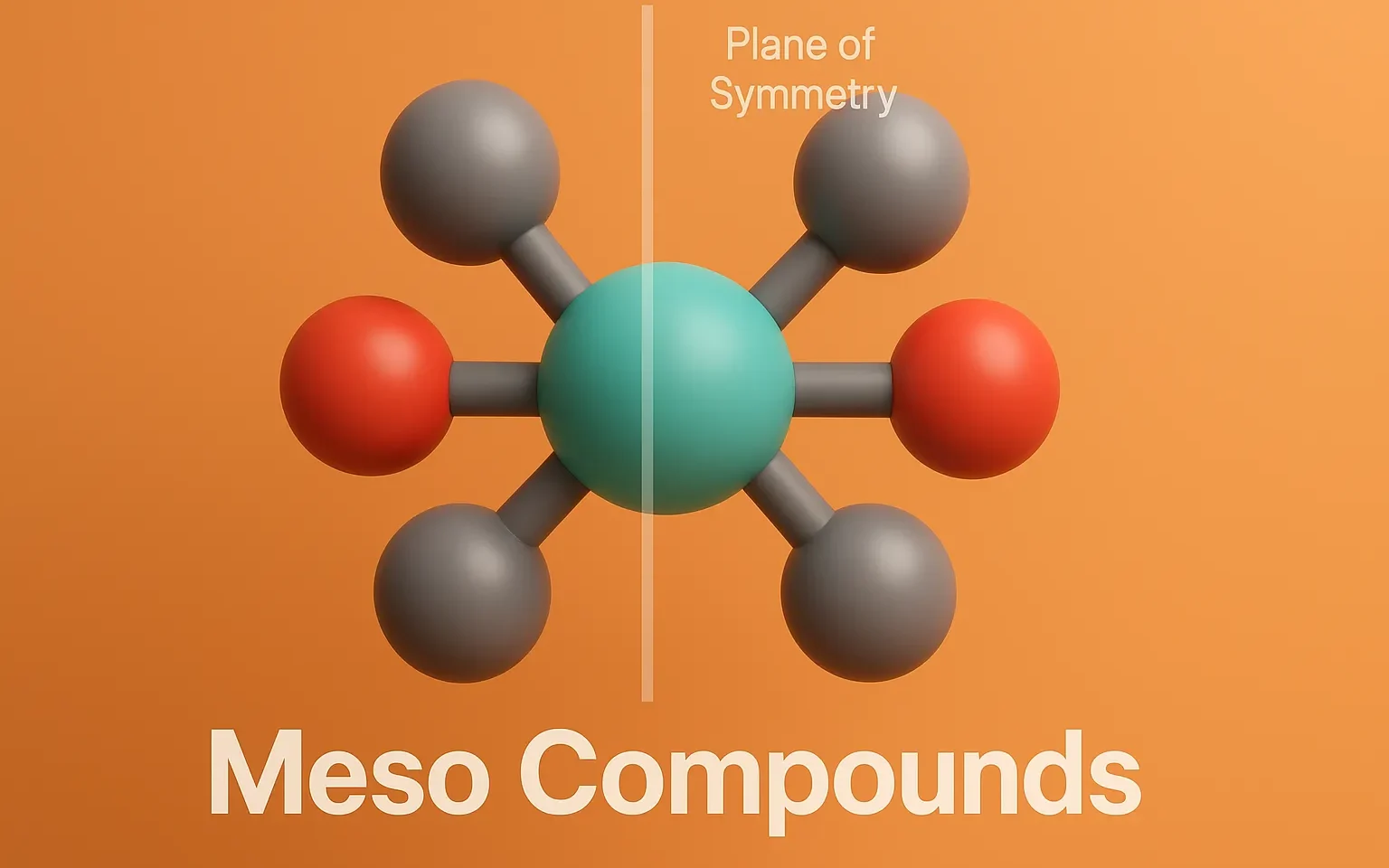
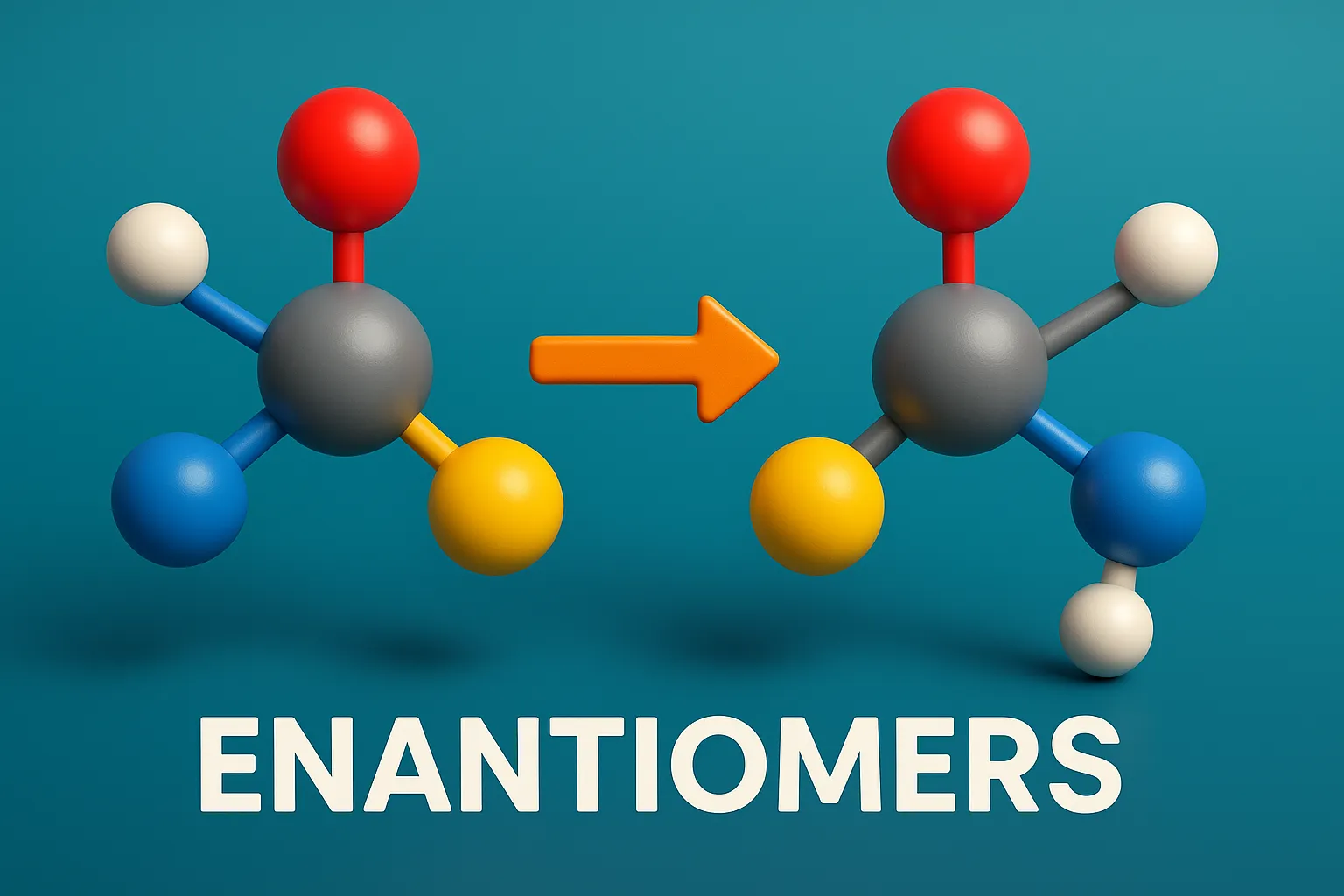
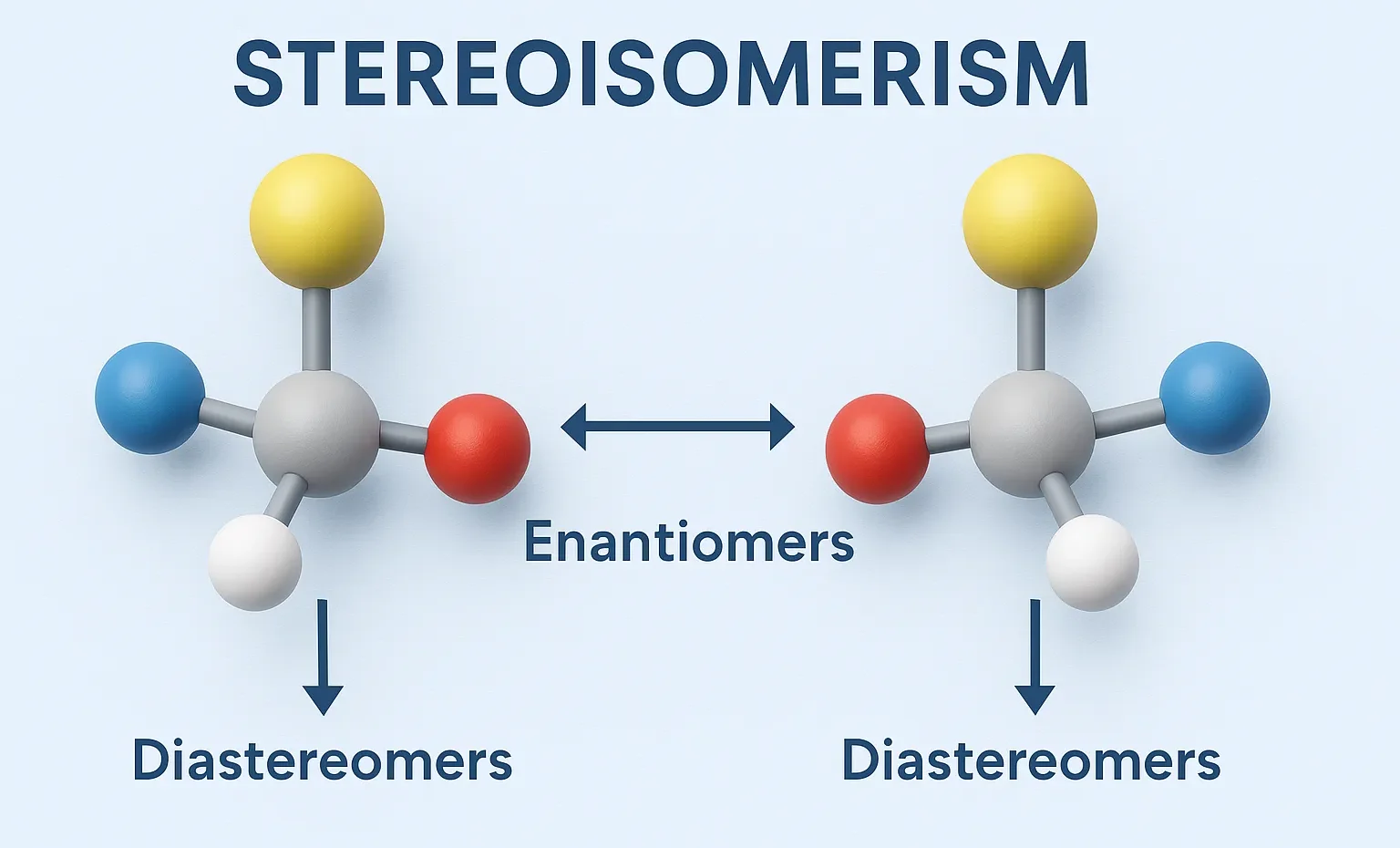
Sequence Rules (Cahn–Ingold–Prelog Rules) assign R or S configuration to chiral centers based on priority of substituent groups. These rules are used to assign priorities to groups around a chiral center when determining R/S configurations. Sequence Rules: Priority by atomic number: Higher atomic number = higher priority. I > Br > Cl > S > … Read more