E1 and E2 Reactions: Kinetics, Reactivity, Carbocation, Rearrangement, Saytzeff’s Rule, and Evidence
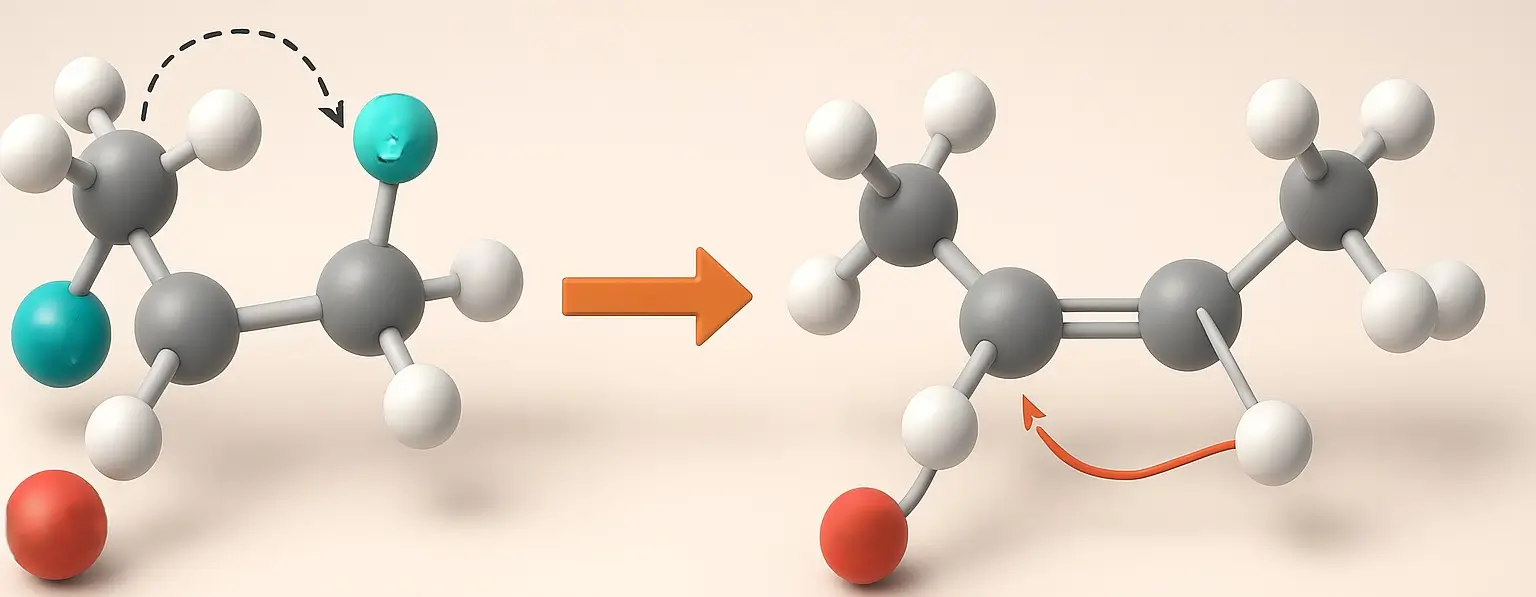
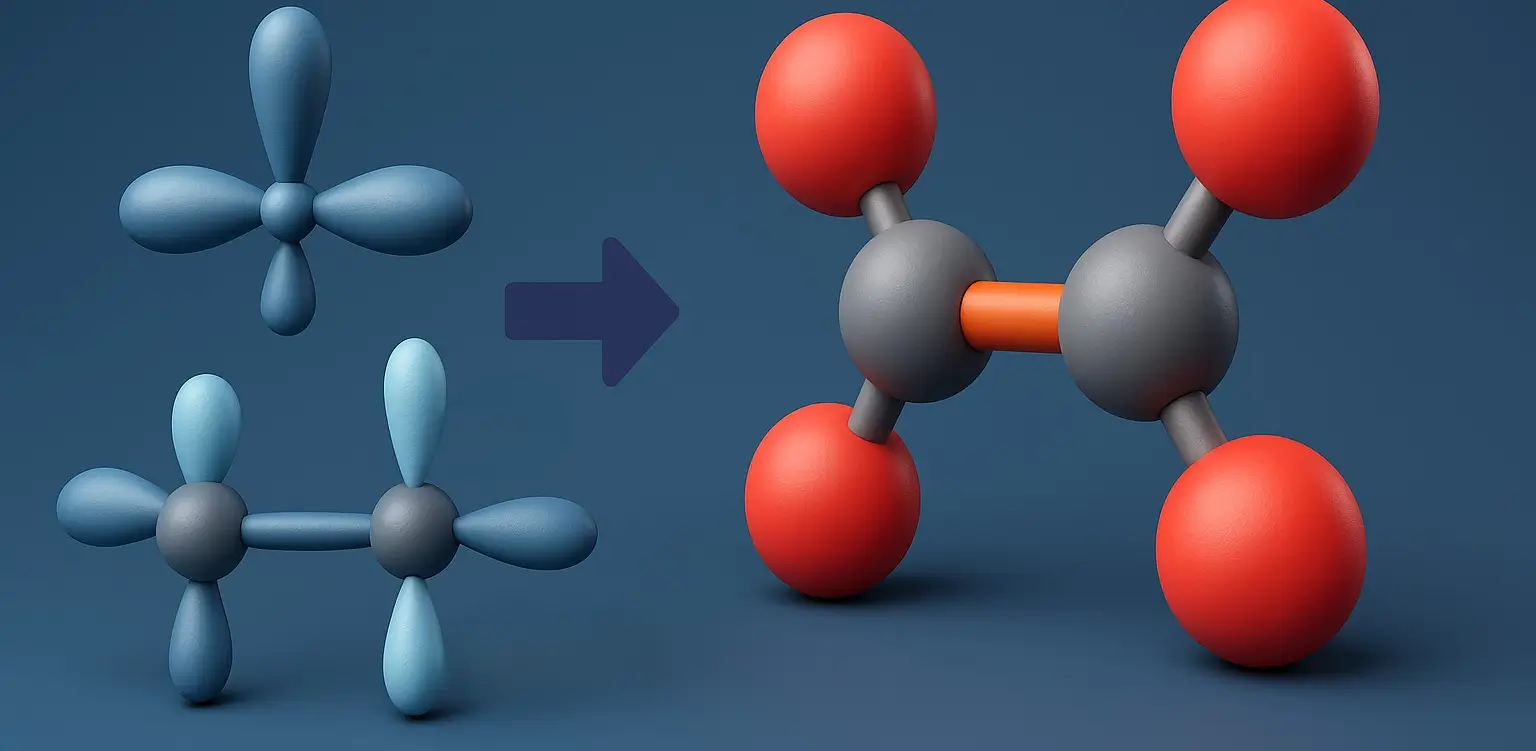
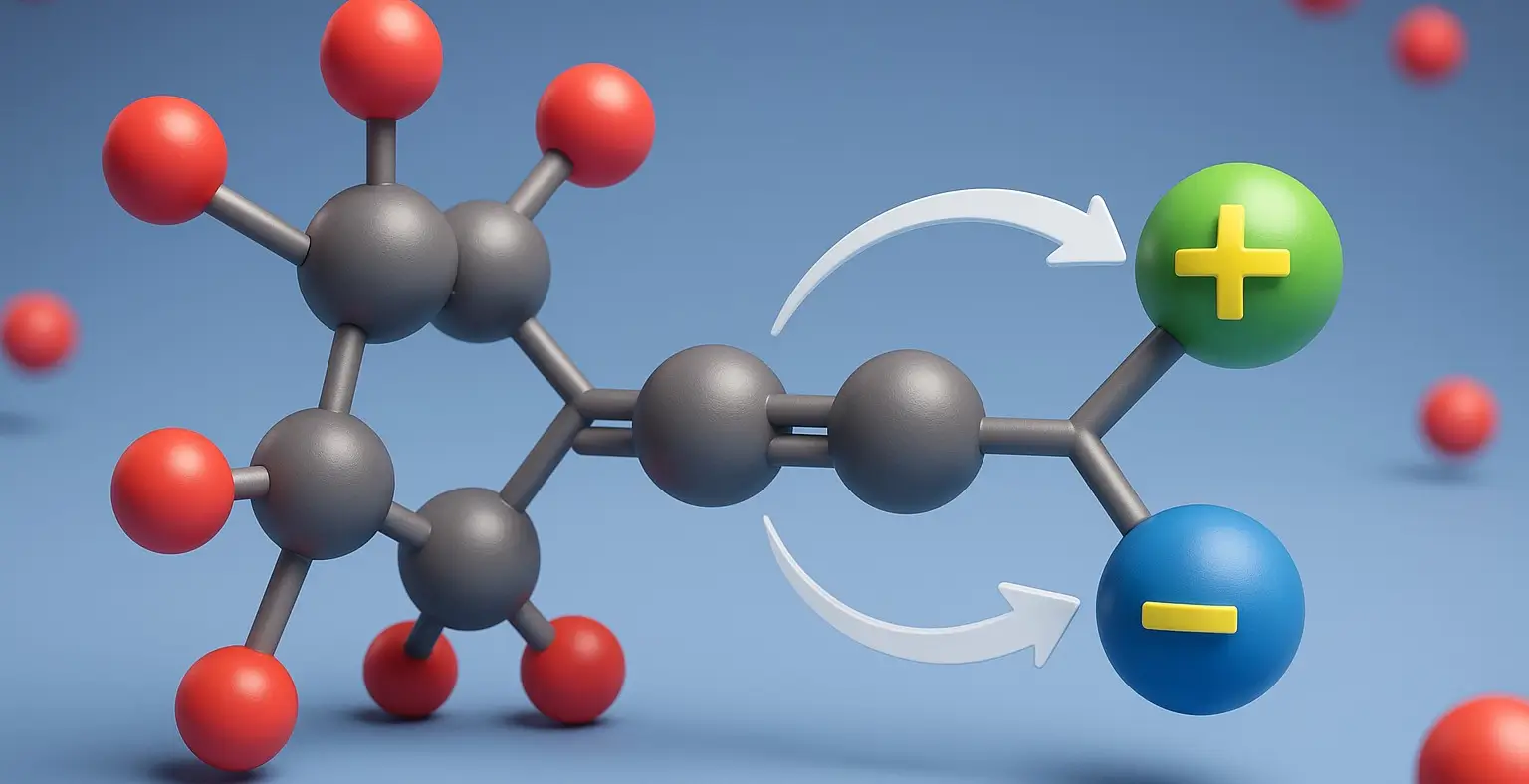
E1 and E2 Reactions Definition E1 (unimolecular) and E2 (bimolecular) are elimination reactions in organic chemistry. E1 occurs in two steps formation of a carbocation followed by proton loss and follows first-order kinetics, favored by weak bases and polar protic solvents. E2 is a one-step reaction where a strong base removes a proton as the … Read more