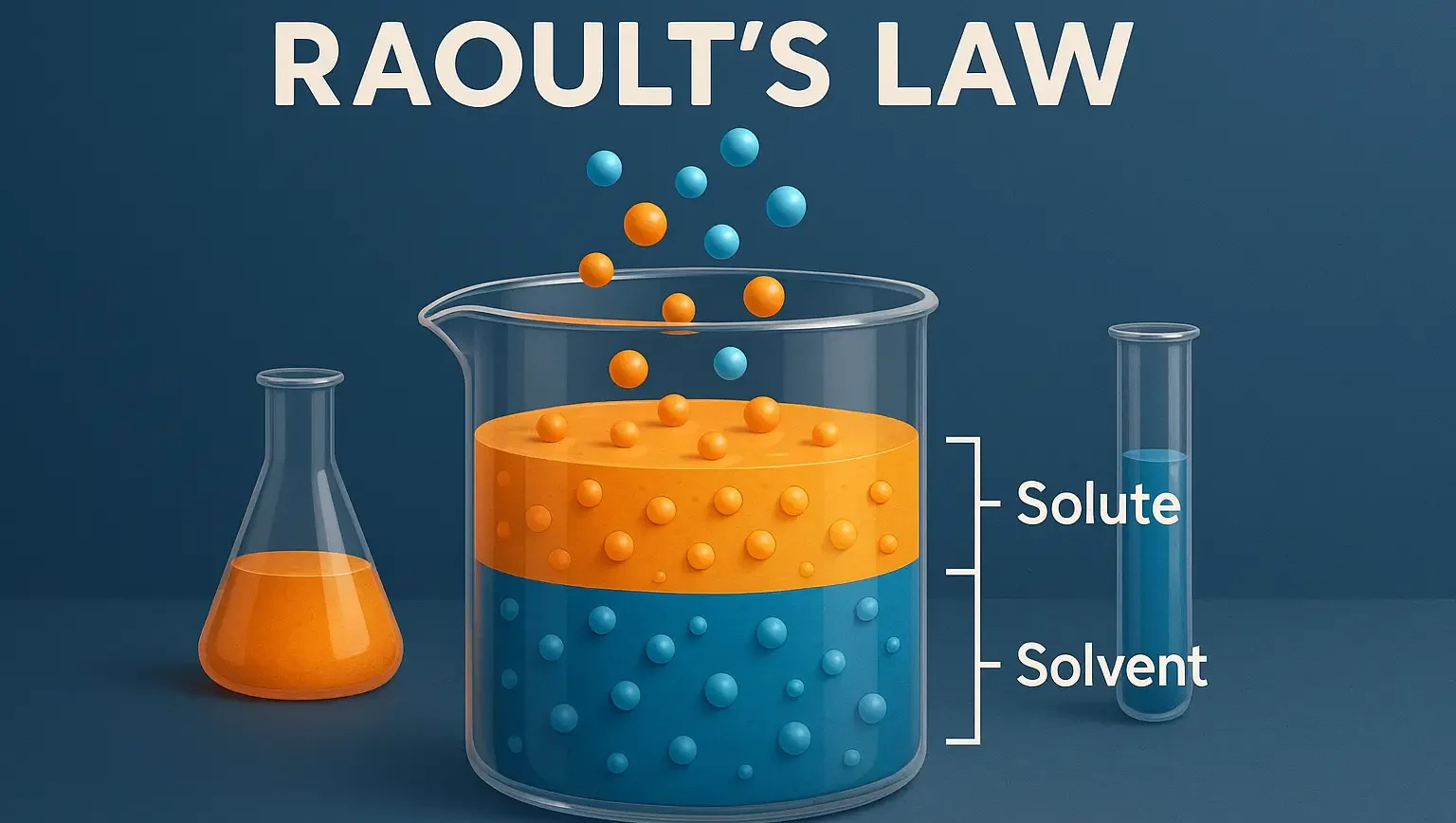
Raoult’s Law & Real Solutions
Raoult’s Law: For an ideal solution, the partial vapor pressure of each component is proportional to its mole fraction in $P_i = X_i \cdot P_i^0$ Pi is the partial vapor pressure of component iii. Xi is the mole fraction of component iii in the liquid phase. Pi0 is the vapor pressure of the pure component … Read more