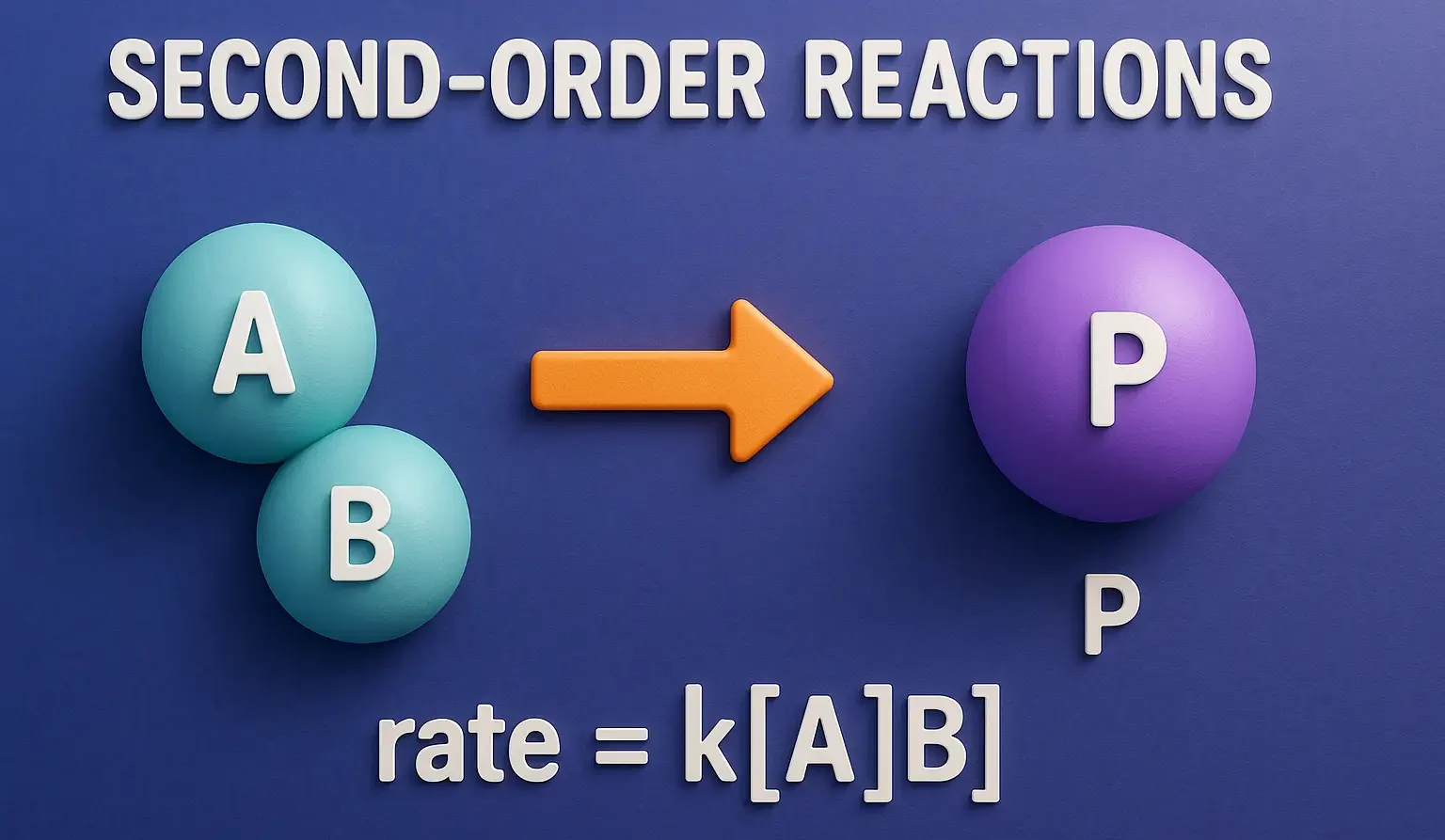
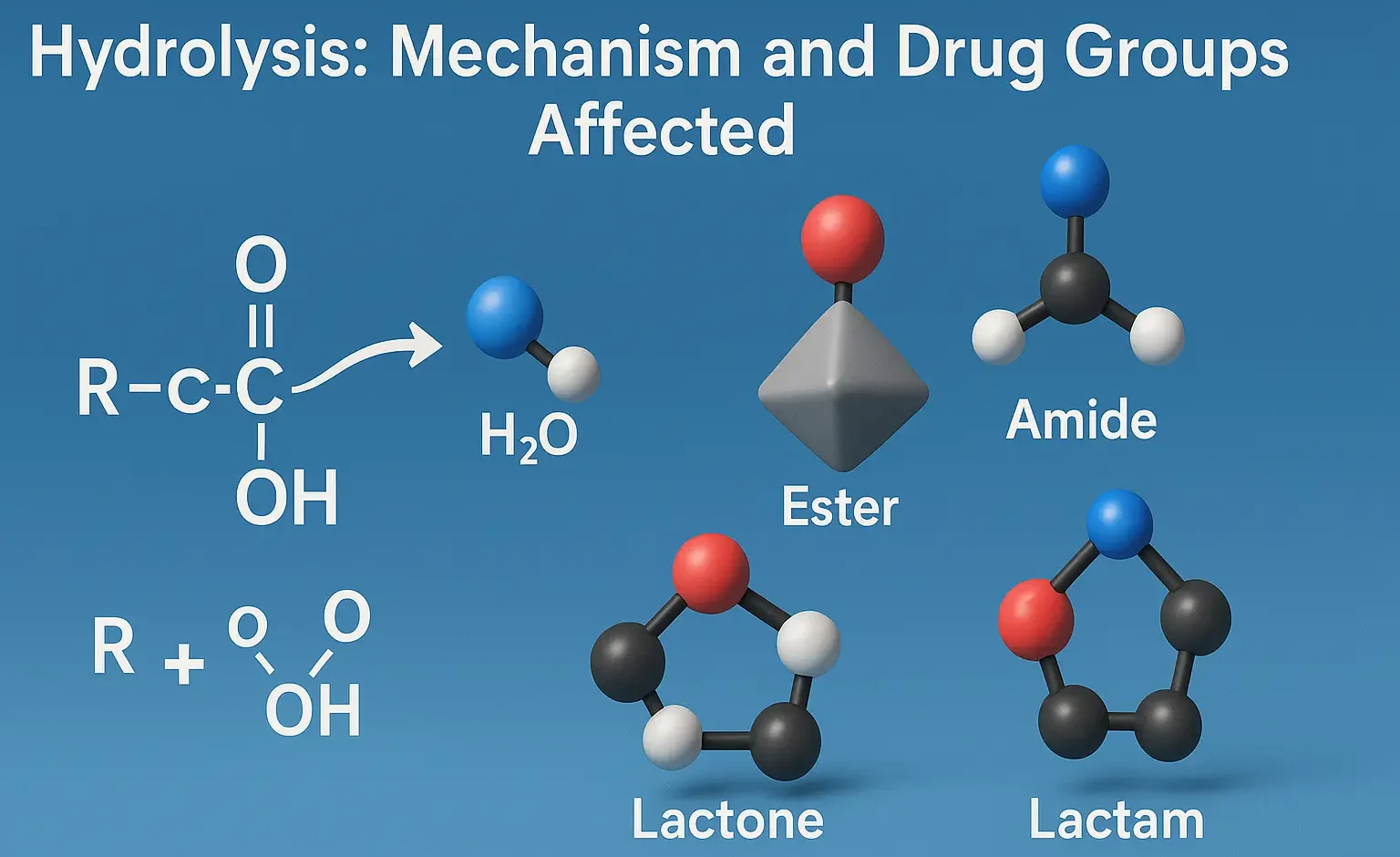
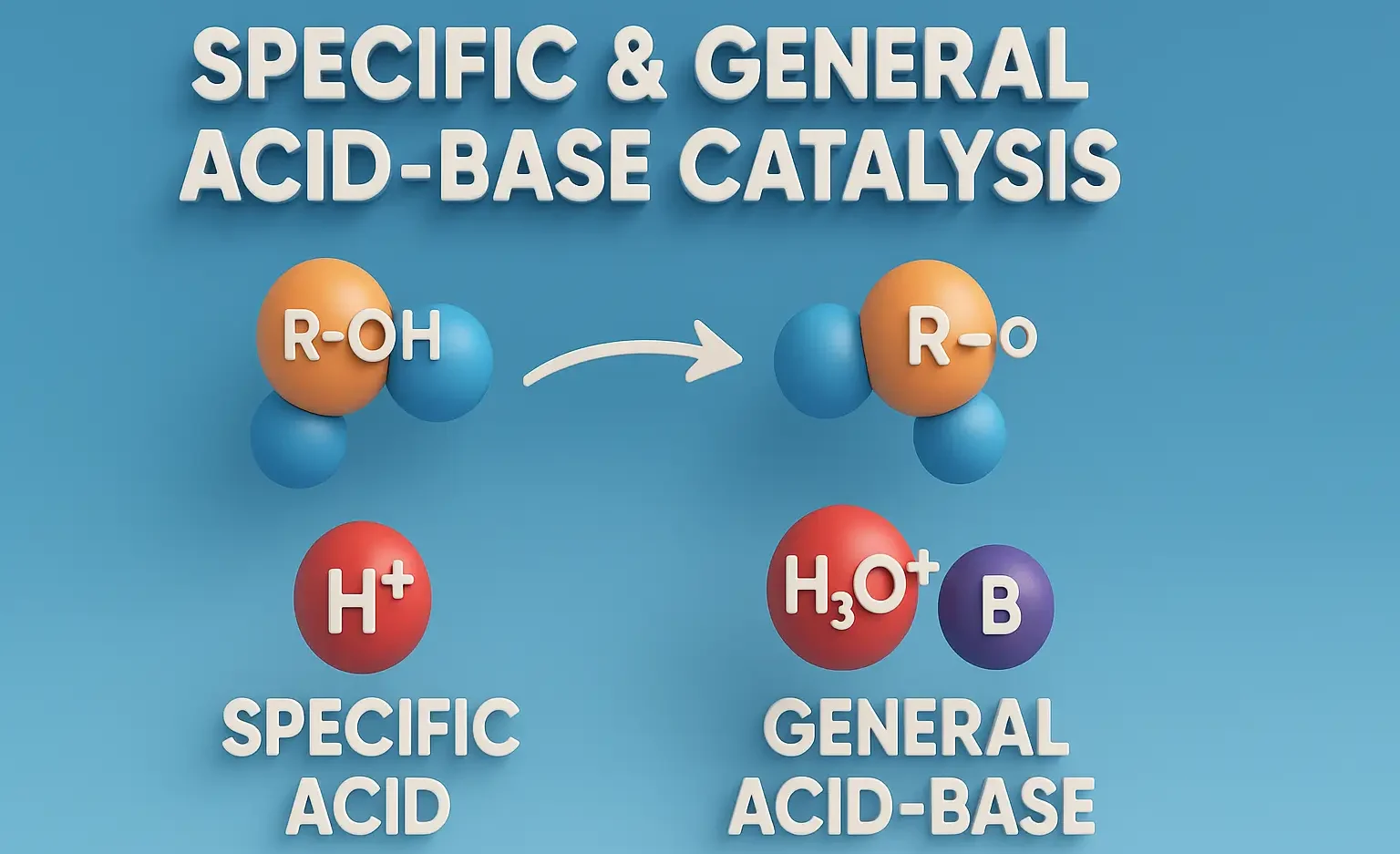
Emulsion Formulation by HLB Method
Emulsion Formulation by HLB Method selects surfactants based on hydrophilic-lipophilic balance for stability. It ensures proper oil-water blending in pharmaceuticals and cosmetics. HLB (Hydrophilic–Lipophilic Balance) is a numerical scale (0–20) used to select appropriate emulsifying agents based on their balance between hydrophilic and lipophilic portions. HLB Scale Interpretation HLB Value Nature of Surfactant Application 1–4 … Read more