Chiral and Achiral Molecules: Chiral molecules have a non-superimposable mirror image due to a chiral center, while achiral molecules are superimposable on their mirror image and lack chirality.
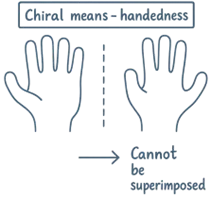
Chiral Molecules
Definition:
- A molecule is chiral if it:
- Has no element of symmetry
- Is not superimposable on its mirror image
- Can rotate plane-polarized light (optically active)
Features:
- Usually contains at least one chiral center (a carbon with four different groups)
- Lacks a plane or center of symmetry
Advertisements
Examples:
- 2-butanol: also has a carbon attached to –H, –CH₃, –CH₂CH₃, and –OH
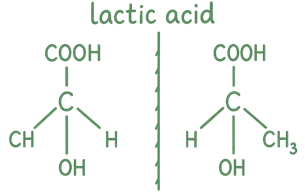
Test for Chirality:
- If you try to superimpose the molecule onto its mirror image and they don’t match, it’s chiral.
Achiral Molecules
Definition:
- A molecule is achiral if it:
- Can be superimposed on its mirror image
- Has at least one element of symmetry
- Is optically inactive

Advertisements
Features:
- May or may not have chiral centers
- Meso compounds are good examples of achiral molecules with chiral centers
Examples:
- Glycine: the simplest amino acid, has two H atoms on the alpha-carbon → achiral
- Meso-tartaric acid: has two chiral centers but is overall achiral due to symmetry
Click Here to Watch the Best Pharma Videos
Advertisements