- Classification of Colloids is based on phase systems like sols, gels, emulsions, and foams.
- Classification of Colloids depends on physical state, interaction, and nature of dispersed phase.
- Colloids can be classified in several ways:
Based on the Physical State of Dispersed and Dispersion Phase:
| Dispersed Phase | Dispersion Medium | Name | Example |
| Solid | Solid | Solid sol | Colored glass |
| Solid | Liquid | Sol | Paints, inks |
| Solid | Gas | Aerosol | Smoke |
| Liquid | Solid | Gel | Cheese, jellies |
| Liquid | Liquid | Emulsion | Milk, cream |
| Liquid | Gas | Aerosol | Fog, mist |
| Gas | Solid | Solid foam | Pumice stone |
| Gas | Liquid | Foam | Whipped cream |
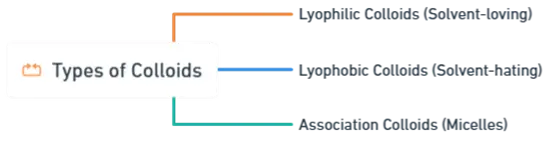
Based on Interaction Between Phases:
Advertisements
1. Lyophilic Colloids (Solvent-loving Colloids)
- Definition:
- Lyophilic colloids are colloidal systems in which the dispersed phase has a strong affinity for the dispersion medium (especially water). “Lyophilic” means “liquid-loving”.
- Examples:
- Gums, gelatin, starch, proteins, cellulose
- Characteristics:
- Formed easily by mixing substances with the dispersion medium.
- Reversible in nature: If the dispersion medium is evaporated, the sol can be remade by simply adding the medium back.
- Highly stable even in the presence of electrolytes.
- Show less tendency to coagulate.
- Exhibit high viscosity and strong hydration.
- Less pronounced Tyndall effect and Brownian movement.
2. Lyophobic Colloids (Solvent-hating Colloids)
- Definition:
- Lyophobic colloids are those in which the dispersed phase has little or no affinity for the dispersion medium. “Lyophobic” means “liquid-hating”.
- Examples:
- Metal sols (gold, silver), metal sulfides (As₂S₃, Sb₂S₃)
- Characteristics:
- Cannot be formed by direct mixing; require special methods (e.g., electrical dispersion, chemical reactions).
- Irreversible in nature: Once precipitated, they cannot be easily re-dispersed.
- Unstable: Can be easily coagulated by small amounts of electrolytes.
- Low viscosity and less hydration.
- Show strong Tyndall effect and vigorous Brownian movement.
- Require protective colloids (often lyophilic) to prevent coagulation.
Advertisements
3. Association Colloids (Micelles)
- Definition:
- These are substances that behave like electrolytes at low concentration but form colloidal solutions at higher concentrations by self-association of molecules.
- These are amphiphilic molecules, having both hydrophilic (water-loving) and hydrophobic (water-hating) parts.
- Examples:
- Soaps (sodium stearate), detergents (sodium lauryl sulfate)
- Micelle Formation:
- Occurs above a certain concentration called the Critical Micelle Concentration (CMC).
- Commonly formed in water, where hydrophobic parts cluster together to avoid water, forming spherical structures (micelles), with hydrophilic heads facing outwards.
- Characteristics:
- Behave like normal electrolytes below CMC.
- Form micelles and behave like colloids above CMC.
- Used in cleaning applications due to ability to trap grease/oil in micelles.
Based on the Nature of Dispersion:
- Multimolecular colloids: Aggregates of small molecules (e.g., gold sol).
- Macromolecular colloids: Single large molecules dispersed (e.g., starch, cellulose).
- Associated colloids (Micelles):
- Surfactant-based colloids above a certain concentration (CMC).
- Exhibit properties of both true solutions and colloids.
- Examples: Soaps, detergents.
Advertisements