The classification of complexation, as shown in the image, is structured into three main categories. Here’s a detailed explanation:
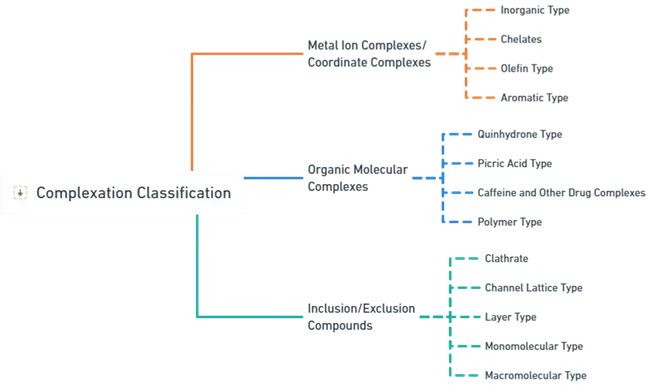
1. Metal Ion Complexes/Coordinate Complexes
- These are complexes formed by metal ions binding with various ligands. This category includes:
- Inorganic Type: These complexes involve metal ions forming bonds with inorganic ligands.
- Chelates: A specific type of complex where metal ions bind with a ligand that has multiple binding sites, creating a ring structure. Chelates are stable due to the formation of multiple bonds with the metal ion.
- Olefin Type: These complexes involve metal ions binding with olefins (unsaturated hydrocarbons containing a carbon-carbon double bond).
- Aromatic Type: Complexes formed when metal ions bind with aromatic compounds, which are compounds containing a conjugated pi-electron system, like benzene.
2. Organic Molecular Complexes
- These complexes involve organic molecules interacting with other compounds. They are further classified into:
- Quinhydrone Type: Complexes formed by quinhydrone, which is a compound consisting of a 1:1 mixture of quinone and hydroquinone.
- Picric Acid Type: Complexes formed with picric acid, which is a nitroaromatic compound that acts as a ligand.
- Caffeine and Other Drug Complexes: These complexes involve drugs like caffeine interacting with other molecules, often forming complexes through hydrogen bonding or other interactions.
- Polymer Type: Complexes involving polymers, which are large molecules composed of repeated subunits, forming bonds with smaller molecules or ions.
Advertisements
3. Inclusion/Exclusion Compounds
- These are complexes where one molecule (the host) forms a cavity or structure that encapsulates another molecule (the guest). This category includes:
- Clathrate: A compound in which molecules are physically trapped within the lattice structure of another compound without any chemical bonding between them.
- Channel Lattice Type: Complexes where the host molecule creates a channel-like structure, allowing the guest molecule to be included.
- Layer Type: These involve layers of host molecules trapping the guest molecules between them.
- Monomolecular Type: Single host molecules encapsulating guest molecules.
- Macromolecular Type: Large host molecules, often macromolecules like cyclodextrins, that include smaller guest molecules within their structure.
- This classification scheme categorizes complexation based on the type of interaction and the nature of the molecules involved.
- It is useful for understanding how different types of complexes are formed in both inorganic and organic chemistry contexts.
Types of Ligands
- Monodentate Ligands: Bind to the central atom through a single donor atom.
- Bidentate Ligands: Have two donor atoms that can bind to the central atom simultaneously.
- Polydentate Ligands: Have three or more donor atoms that can attach to the central atom.
- Chelating Ligands: Form one or more rings by binding to the central atom through multiple donor atoms.
- Ambidentate Ligands: Have two or more donor atoms, but only one binds to the central atom at a time.
- Bridging Ligands: Simultaneously bind to two or more central atoms, forming a bridge between them.

