Definition:
- Dispersed systems consist of two phases:
- Dispersed phase (internal phase): The substance that is dispersed.
- Dispersion medium (continuous phase): The substance in which the dispersed phase is distributed.
Classification of Dispersed Systems:
- Dispersed systems are classified based on:
- Particle size of the dispersed phase
- Physical state of both the dispersed phase and dispersion medium
Advertisements
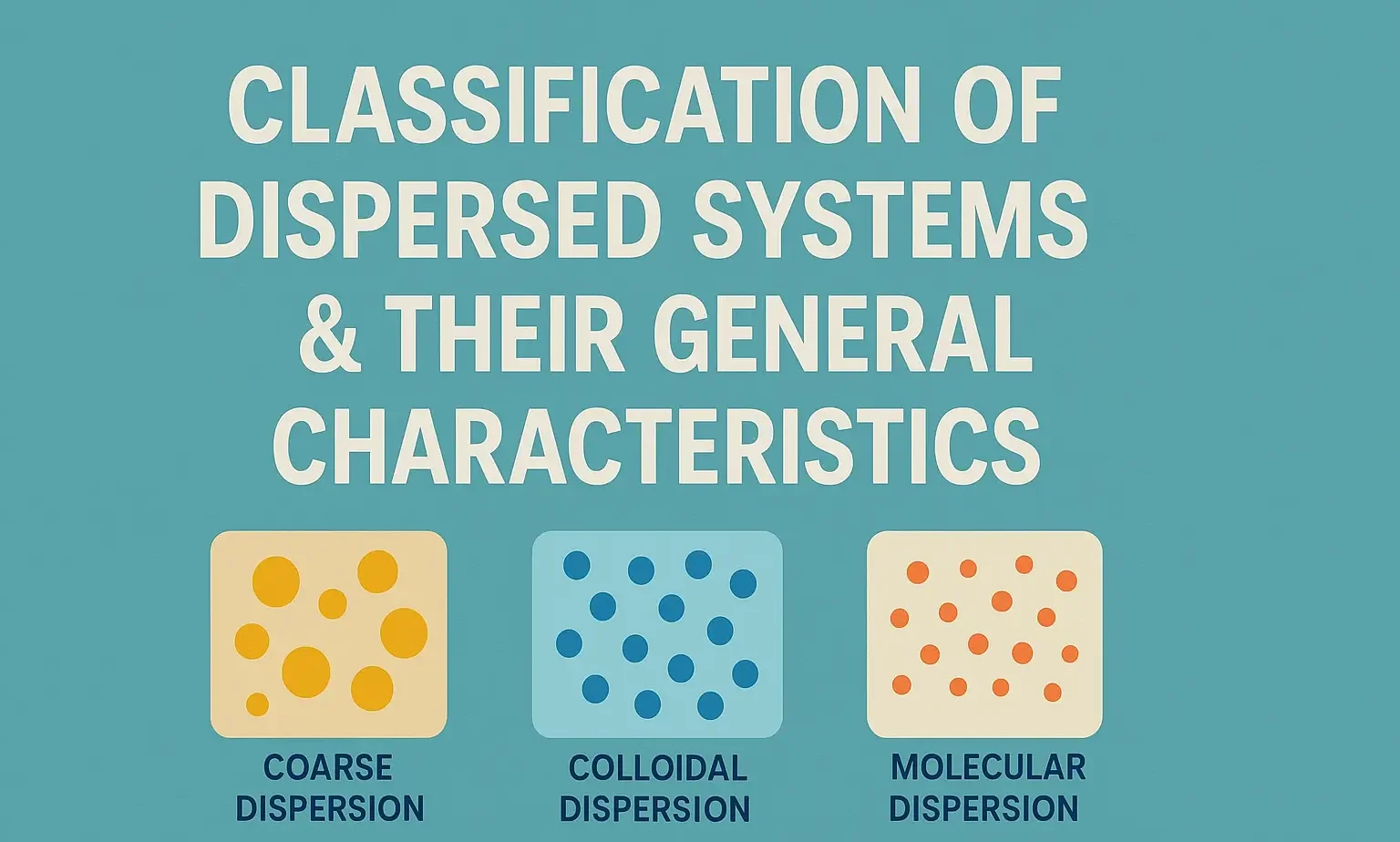
1. Based on Particle Size
| Type | Particle Size Range | Example |
| Molecular (True) Solutions | < 1 nm | NaCl in water |
| Colloidal Dispersions | 1 – 1000 nm (1 nm to 1 µm) | Milk, gels, magmas |
| Coarse Dispersions | > 1 µm | Suspensions, emulsions |
2. Based on Physical States (Phases)
There are 8 types depending on the physical state of dispersed phase and dispersion medium.
| Dispersed Phase | Dispersion Medium | Type | Example |
| Solid | Gas | Solid aerosol | Smoke, airborne particles |
| Liquid | Gas | Liquid aerosol | Mist, fog, sprays |
| Gas | Liquid | Foam | Shaving cream, whipped cream |
| Solid | Liquid | Sol | Paints, gold sol |
| Liquid | Liquid | Emulsion | Milk, creams |
| Gas | Solid | Solid foam | Pumice, foam rubber |
| Liquid | Solid | Gel | Gelatin, jelly |
| Solid | Solid | Solid sol | Colored glass, pearls |
General Characteristics of Dispersed Systems:
- Heterogeneous nature: Composed of two distinct phases.
- Stability: Varies from very stable (true solutions) to unstable (coarse dispersions).
- Visibility: Particles in colloidal systems are not visible to the naked eye but can be detected under an ultramicroscope.
- Filterability: Colloidal particles can pass through ordinary filters but not through ultrafilters.
- Brownian motion: Colloidal particles exhibit random movement due to collisions with dispersion medium molecules.
- Tyndall effect: Scattering of light by colloidal particles, making the path of light visible.
Advertisements