Clinical trial phase evaluates drug safety, dosage, and effectiveness in humans through structured studies.
Clinical Trial Phase
Involves systematic testing in humans, divided into several phases:
Phase 0 (Optional):
- Microdosing studies in a few volunteers
- Assesses basic pharmacokinetics and drug-target interaction
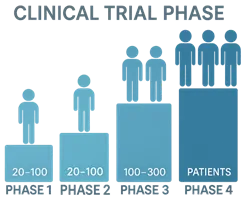
Phase I:
- Conducted in healthy volunteers (20–100)
- Focuses on safety, tolerability, and pharmacokinetics
- Determines the maximum tolerated dose
Phase II:
- Conducted in patients with the target disease (100–300)
- Assesses efficacy, optimal dose, and short-term side effects
Phase III:
- Large-scale studies (1,000–3,000 or more patients)
- Confirms effectiveness, compares with standard treatments
- Monitors for rare or long-term side effects
- Data used for submitting New Drug Application (NDA)
Phase IV (Post-Marketing Surveillance):
- Conducted after drug approval and marketing
- Evaluates long-term safety, efficacy, and detection of rare adverse effects
- Helps refine dosing and usage recommendations
Click Here to Watch the Best Pharma Videos

