- Coacervation is the separation of colloids into two liquid phases, rich and poor in dispersed particles.
- It is used in microencapsulation, pharmaceuticals, and controlled drug delivery systems.
Definition of Coacervation:
- A phase separation process where colloids separate into two liquid phases:
- Dispersed phase (coacervate) – rich in colloidal material
- Equilibrium phase – depleted in colloid
Types:
- Simple Coacervation: Caused by adding salts, changing pH or temperature.
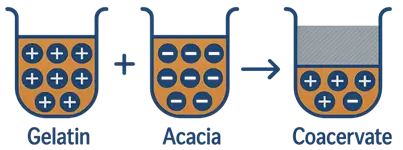
- Complex Coacervations: Involves two oppositely charged colloids (e.g., gelatin + gum arabic).
Cause:
- Addition of a third substance (e.g., polymer or salt), or change in pH or temperature.
Advertisements
Applications in Pharmaceutics:
- Microencapsulation: Used to coat drugs, allowing for:
- Controlled release
- Taste masking
- Protection from environment (light, moisture)