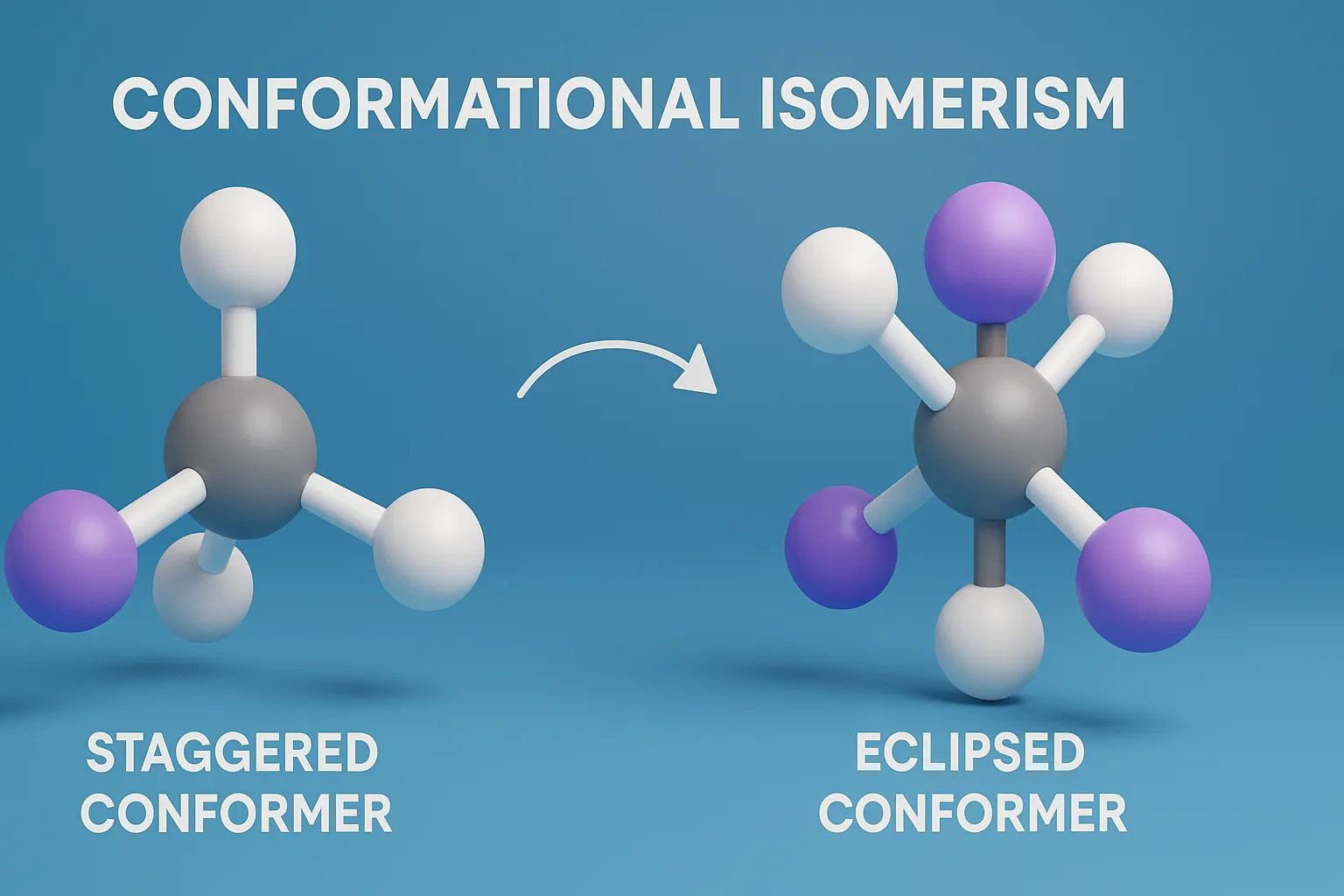
Conformational Isomerism is a type of stereoisomerism that arises from rotation around single covalent bonds, producing different spatial arrangements of atoms without breaking bonds. These conformers, such as staggered, eclipsed, or gauche forms, often differ in stability and energy, influencing the physical and chemical properties of molecules.
Definition of Conformational Isomerism
- Conformational isomers (or conformers) are different spatial arrangements of a molecule’s atoms that result from rotation around single (sigma) bonds.
- They do not involve breaking bonds.
- They interconvert rapidly at room temperature.
- Energy differences arise due to:
- Torsional strain: Electron repulsion between eclipsed bonds.
- Steric strain: Repulsion between bulky groups being too close.