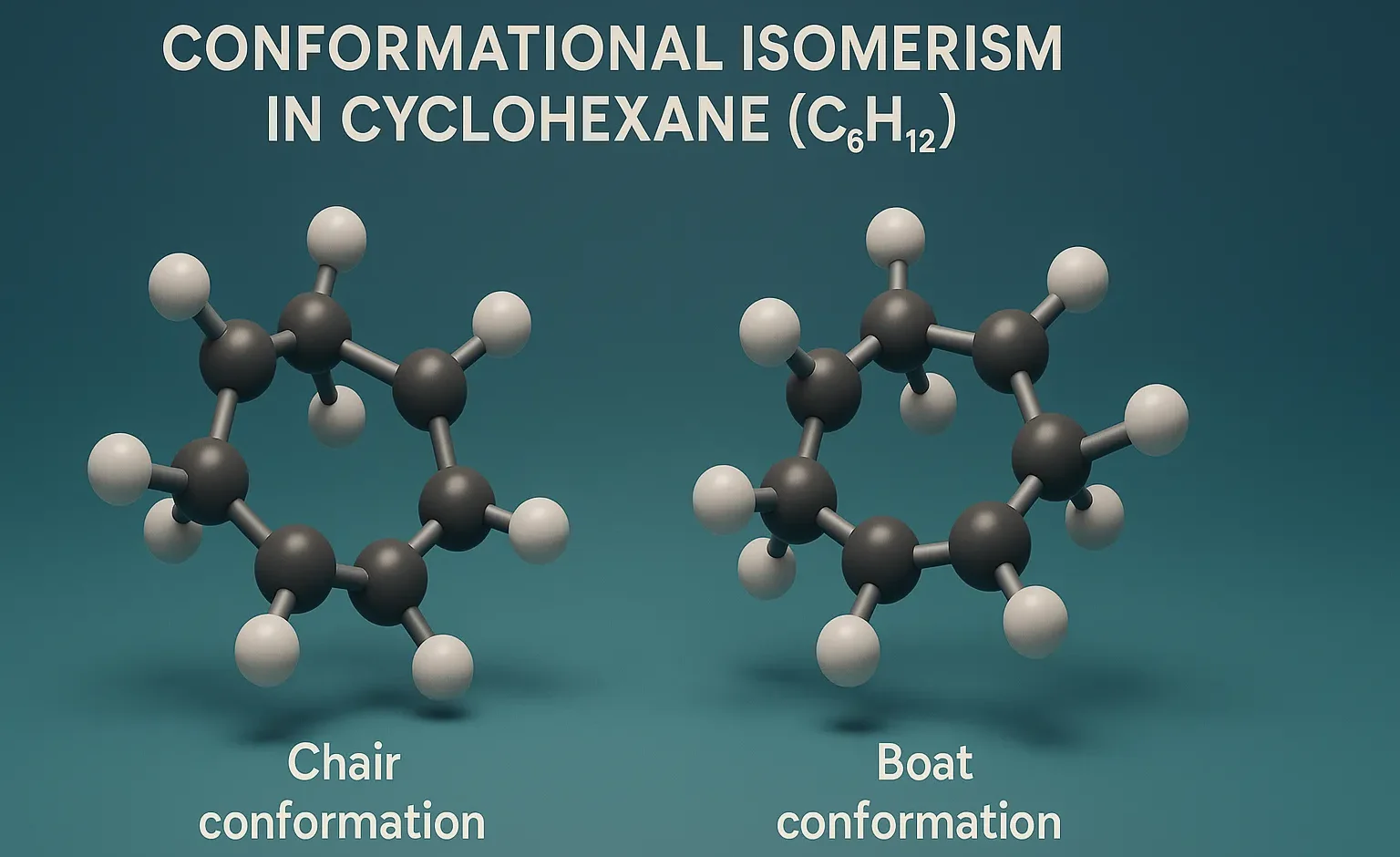
Conformational Isomerism in Cyclohexane
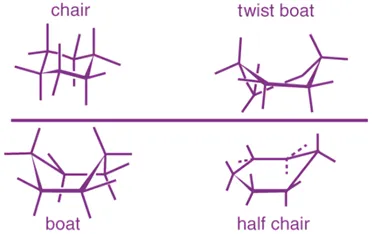
Conformational Isomerism in Cyclohexane (C₆H₁₂) involves chair, boat, twist, and half-chair forms, with the chair conformation being the most stable.
Why Is Cyclohexane Special?
- Although cyclohexane forms a ring, it avoids angle strain (unlike smaller rings) by adopting non-planar conformations.
- The ideal bond angle (109.5°) is maintained through specific 3D shapes, minimizing strain.
Advertisements
Main Conformations
-
Chair Conformation:
- Most stable.
- Bond angles are ~109.5° (ideal tetrahedral).
- All C–H bonds are staggered.
- Hydrogens alternate between axial (up/down) and equatorial (around the ring) positions.
-
Boat Conformation:
- Less stable due to:
- Steric strain (flagpole interactions between axial Hs on C1 and C4).
- Torsional strain (some eclipsing).
- Less stable due to:
-
Twist-Boat Conformation:
- Intermediate in stability.
- Some relief of flagpole and torsional strain.
- More stable than boat, less than chair.
Advertisements
Chair Flip
- Cyclohexane can undergo a chair flip:
- Axial positions become equatorial and vice versa.
- Important when evaluating substituted cyclohexanes.
Substituent Preference
- Large groups prefer the equatorial position to reduce 1,3-diaxial interactions.
- Example: In methylcyclohexane, the equatorial CH₃ is more stable than axial by ~7.6 kJ/mol.
Advertisements