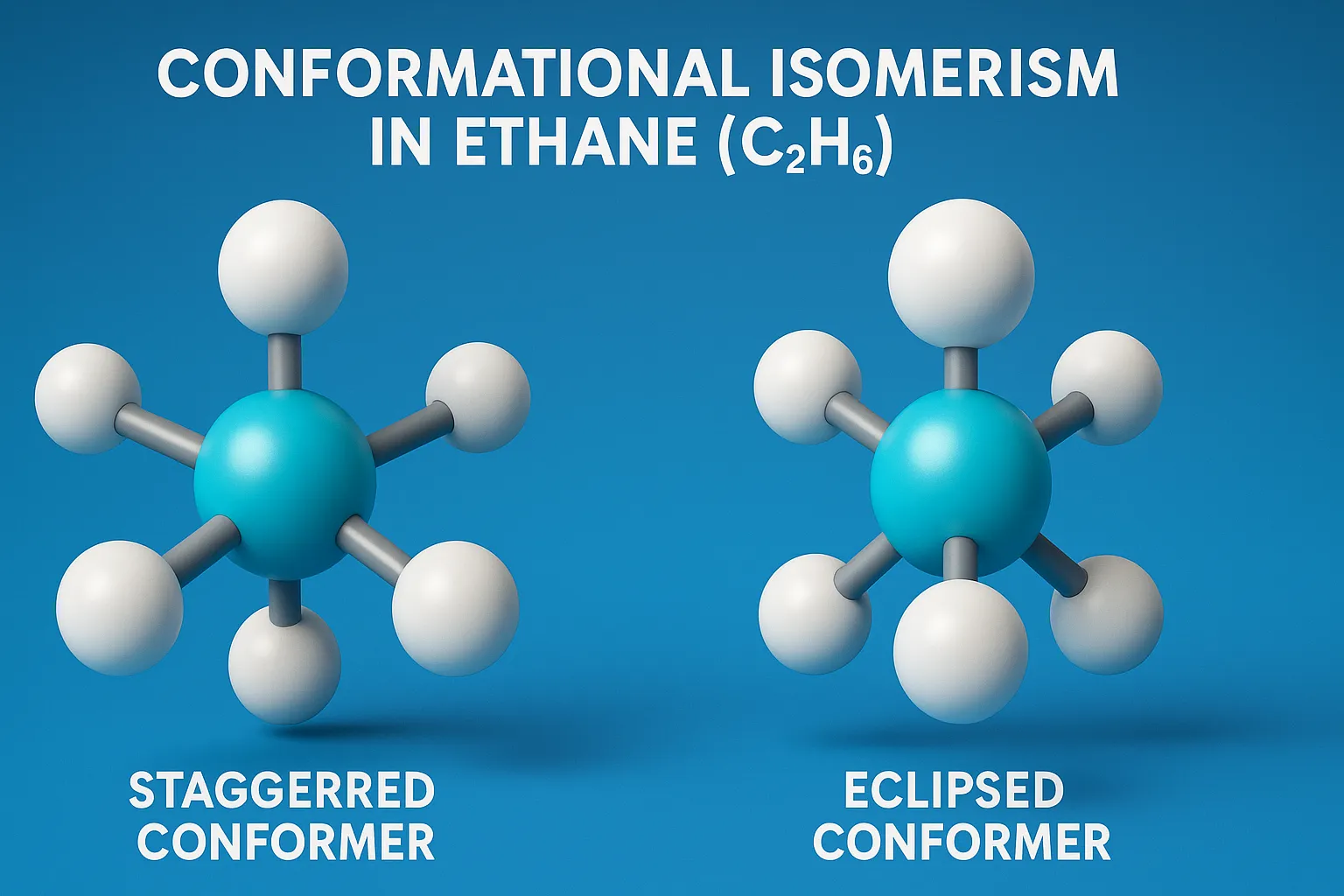
Conformational Isomerism in Ethane (C₂H₆)
Conformational Isomerism in Ethane (C₂H₆) shows staggered and eclipsed forms from C–C bond rotation, with staggered being more stable.
Structure and Rotation
- Ethane contains a C–C sigma bond that allows free rotation.
- Each carbon is bonded to three hydrogen atoms.
- The rotation around the C–C bond leads to different conformations.
Advertisements
Main Conformations
-
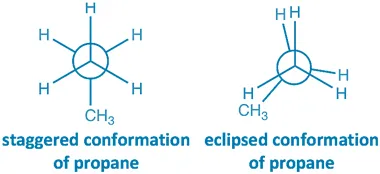
Staggered Conformation:
- The hydrogen atoms on the front and back carbons are 60° apart.
- Torsional strain is minimized.
- Most stable conformation.
-
Eclipsed Conformation:
- The hydrogen atoms are aligned with each other.
- Torsional strain is at maximum due to electron repulsion.
- Least stable conformation.
Advertisements
Advertisements
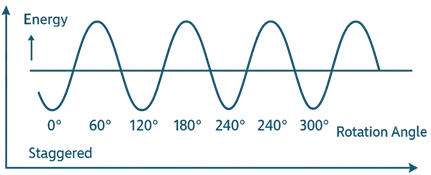
Energy Difference
- The staggered conformation is approximately 12 kJ/mol more stable than the eclipsed conformation.
Potential Energy Diagram
- A sinusoidal curve where:
- Valleys = staggered conformations (lowest energy).
- Peaks = eclipsed conformations (highest energy).
Advertisements