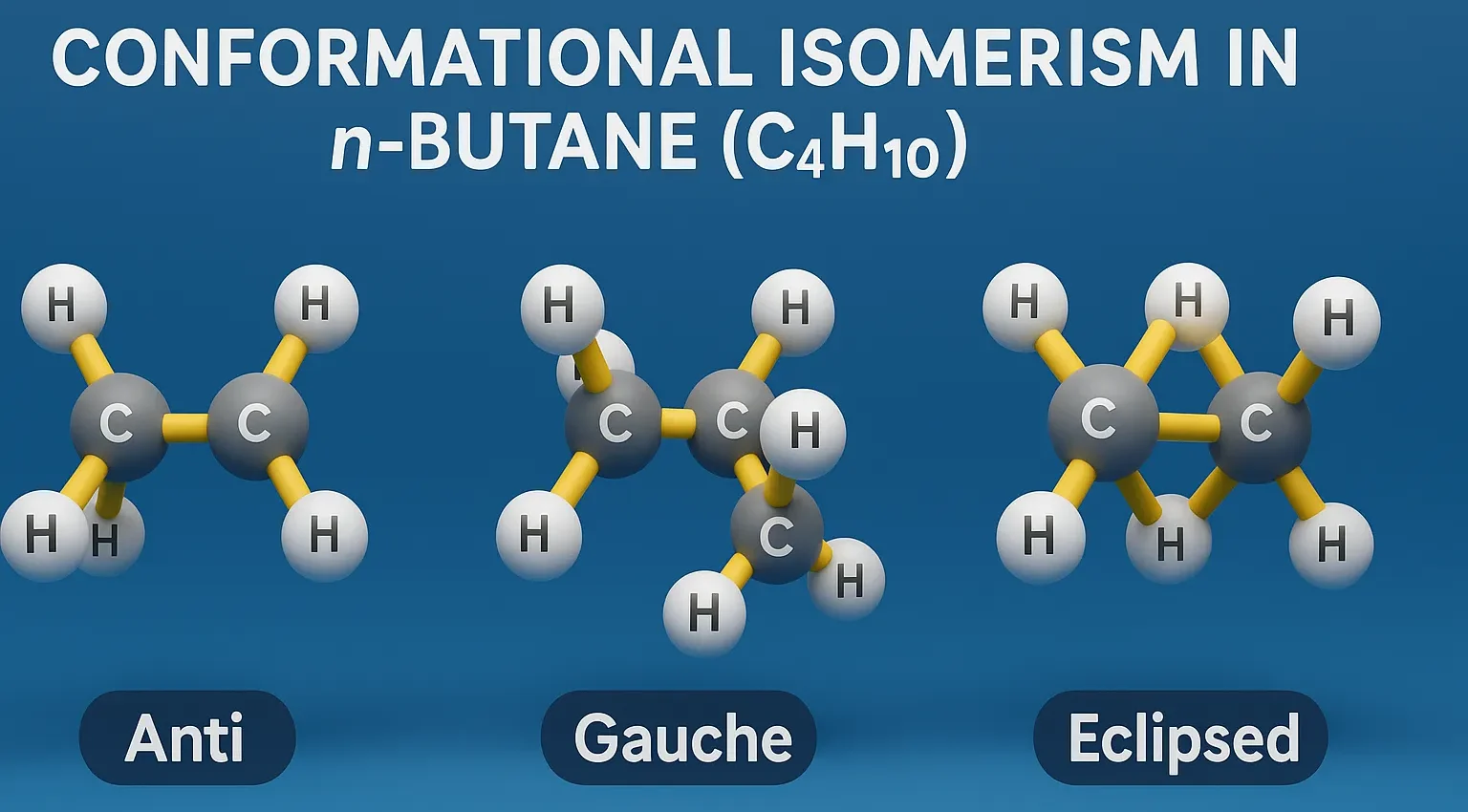
Conformational Isomerism in n-Butane (C₄H₁₀)
Conformational Isomerism in n-Butane (C₄H₁₀) arises from C–C bond rotation, giving anti, gauche, and eclipsed forms with different stabilities.
Rotation around the C₂–C₃ Bond
- n-Butane has a straight chain: CH₃–CH₂–CH₂–CH₃.
- Rotation about the C₂–C₃ bond generates multiple conformations.
Advertisements
Key Conformations
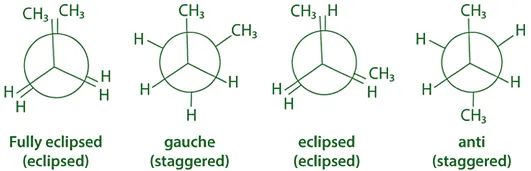
-
Anti Conformation (Staggered):
- The two methyl groups are 180° apart.
- Most stable due to minimal steric hindrance.
-
Gauche Conformation (Staggered):
- Methyl groups are 60° apart.
- Less stable than anti due to steric strain between CH₃ groups.
-
Eclipsed (H–CH₃):
- Methyl on one carbon is aligned with a hydrogen on the other.
- Higher energy than gauche.
-
Fully Eclipsed (CH₃–CH₃):
- Methyl groups are aligned.
- Highest energy due to maximum steric repulsion.
Advertisements
Stability Order
Anti > Gauche > Eclipsed (H–CH₃) > Fully Eclipsed (CH₃–CH₃)
Energy Difference
- Anti to Gauche: ~3.8 kJ/mol
- Anti to Fully Eclipsed: ~18–19 kJ/mol
Advertisements
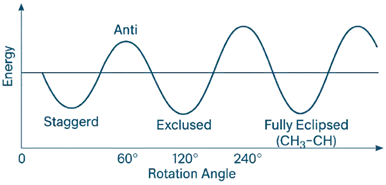
Potential Energy Diagram
- A full 360° rotation around the C₂–C₃ bond produces a sinusoidal energy profile with:
- Minima at staggered conformations (especially anti and gauche)
- Maxima at eclipsed conformations (especially fully eclipsed)
Click Here to Watch the Best Pharma Videos
Advertisements