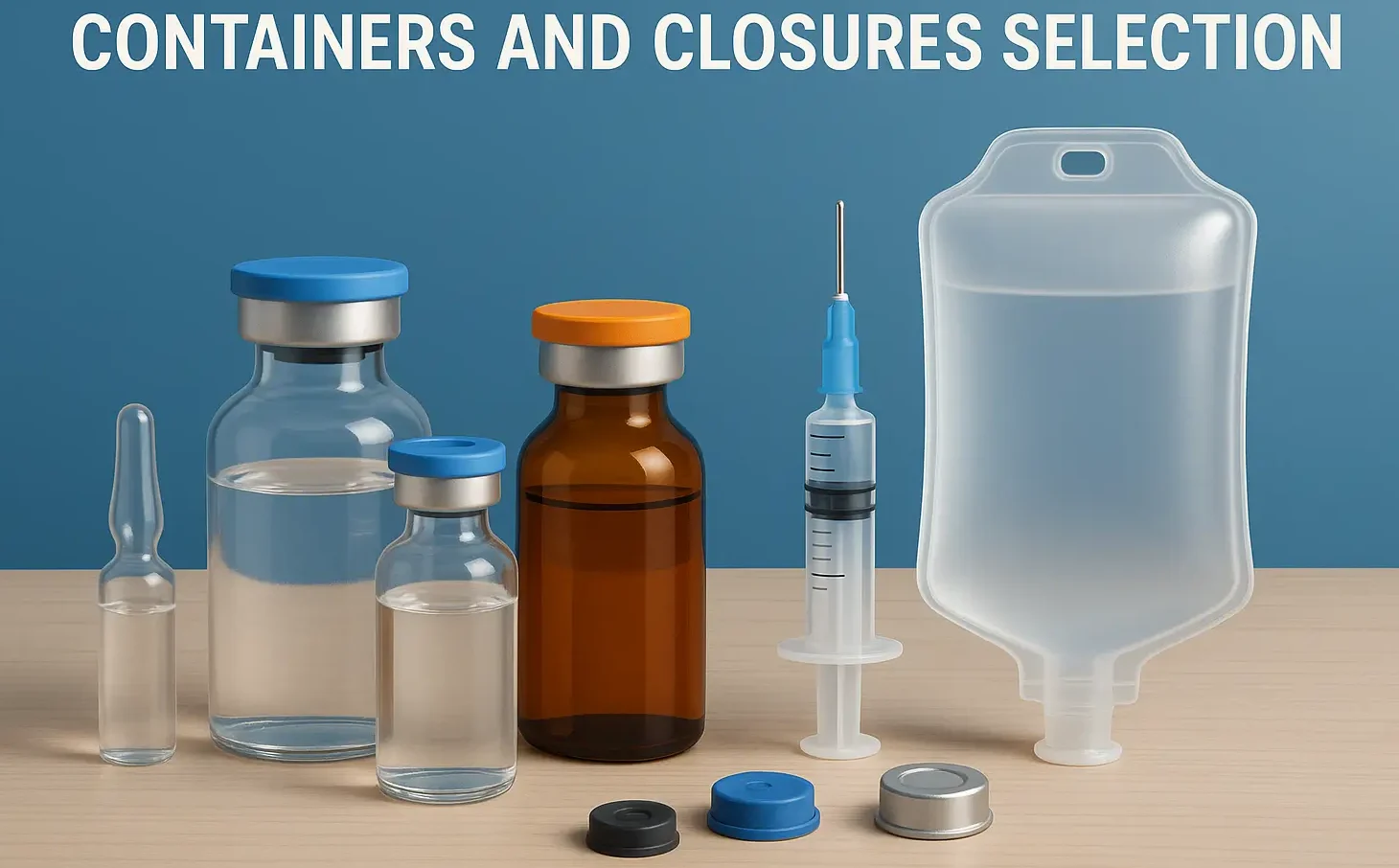
- Containers and Closures Selection plays a critical role in ensuring product stability, sterility, and compatibility for parenteral preparations.
- Containers and Closures Selection also impacts packaging integrity, patient safety, and regulatory compliance in pharmaceutical manufacturing.
Selection Criteria
- Material Compatibility: Containers and closures must not interact chemically with the formulation.
- Sterility: They should be capable of maintaining sterility throughout the product’s shelf life.
- Physical and Chemical Properties: Resistance to breakage, moisture, light, and temperature fluctuations.
- Ease of Administration: Containers should allow easy withdrawal of the product or direct administration.
- Regulatory Compliance: Containers and closures must comply with pharmacopeial and regulatory guidelines.
Types of Containers

-
Glass Containers:
-
Plastic Containers:
- Used for infusion fluids (e.g., flexible plastic bags).
- Made of materials like polypropylene or polyethylene.
- Advantages:
- Lightweight, shatterproof, and flexible.
- Disadvantages:
- Potential for leaching of plasticizers.
Types of Closures
-
Rubber Stoppers:
- Used in vials.
- Composed of butyl, silicone, or natural rubber.
- Should be inert and elastic for proper sealing.
-
Flip-Off Caps and Aluminum Seals:
- Provide tamper-evidence for vials.
-
Sealing Membranes:
- Used in flexible plastic bags.
Click Here to Watch the Best Pharma Videos!