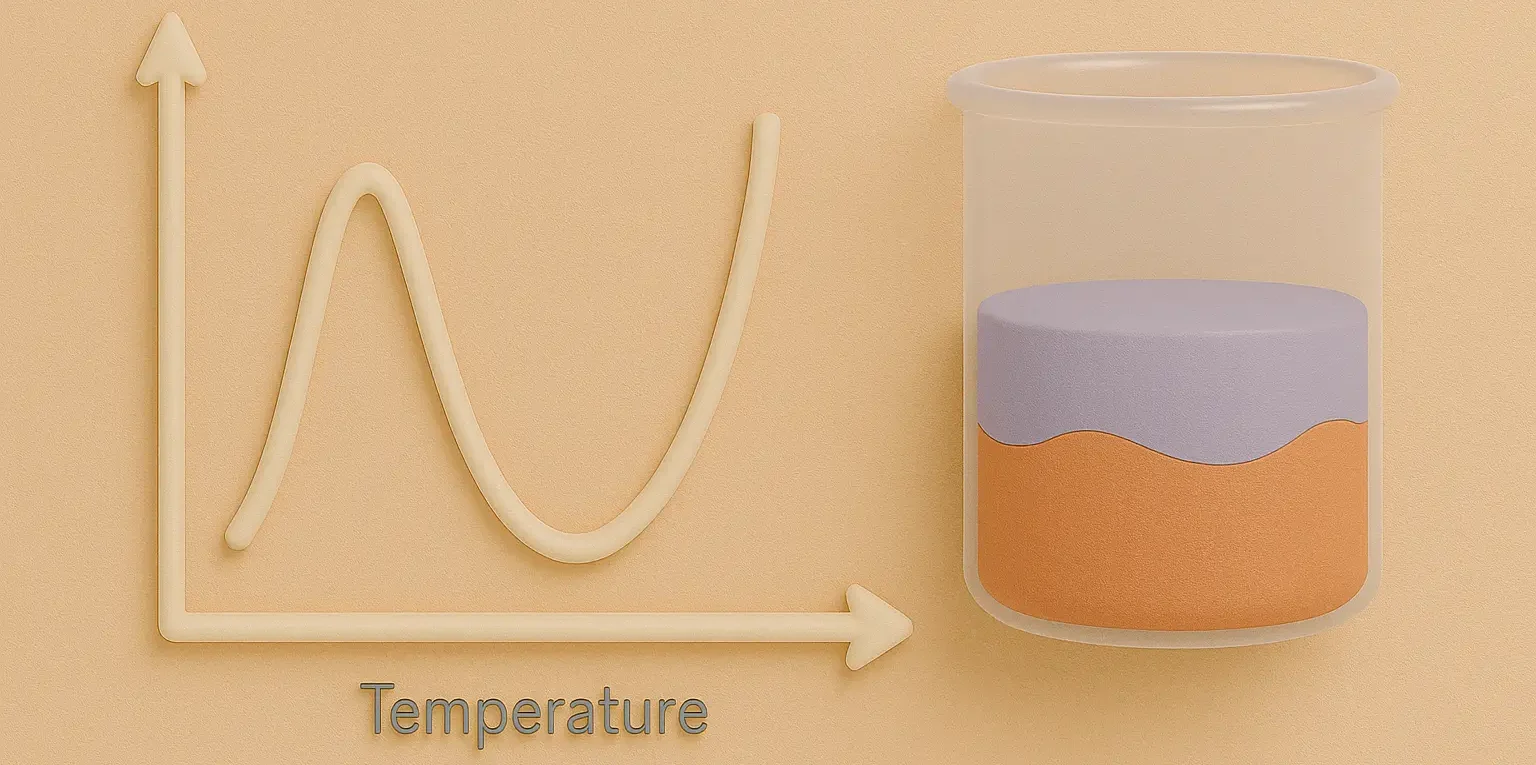
Definition:
The temperature at which two partially miscible liquids become fully miscible (UCST) or fully immiscible (LCST).
-
Upper Critical Solution Temperature (UCST):
- Below this temperature, the liquids are completely miscible.
-
Lower Critical Solution Temperature (LCST):
- Above this temperature, the liquids are completely miscible.
Characteristics:
- Phase Transition Point: Represents the temperature at which the nature of the miscibility changes dramatically.
- Dependency: CST can depend on the nature of the substances, pressure, and the presence of other solutes.
- Examples: Triethylamine and water show UCST behavior, while nicotine and water exhibit an LCST.
Applications:
- Thermosensitive Polymers: Used in temperature-responsive drug delivery systems.
- Chemical Engineering: Important for temperature-controlled processes like solvent recovery.
- Research and Development: Essential in synthesizing new materials with desired thermal properties.