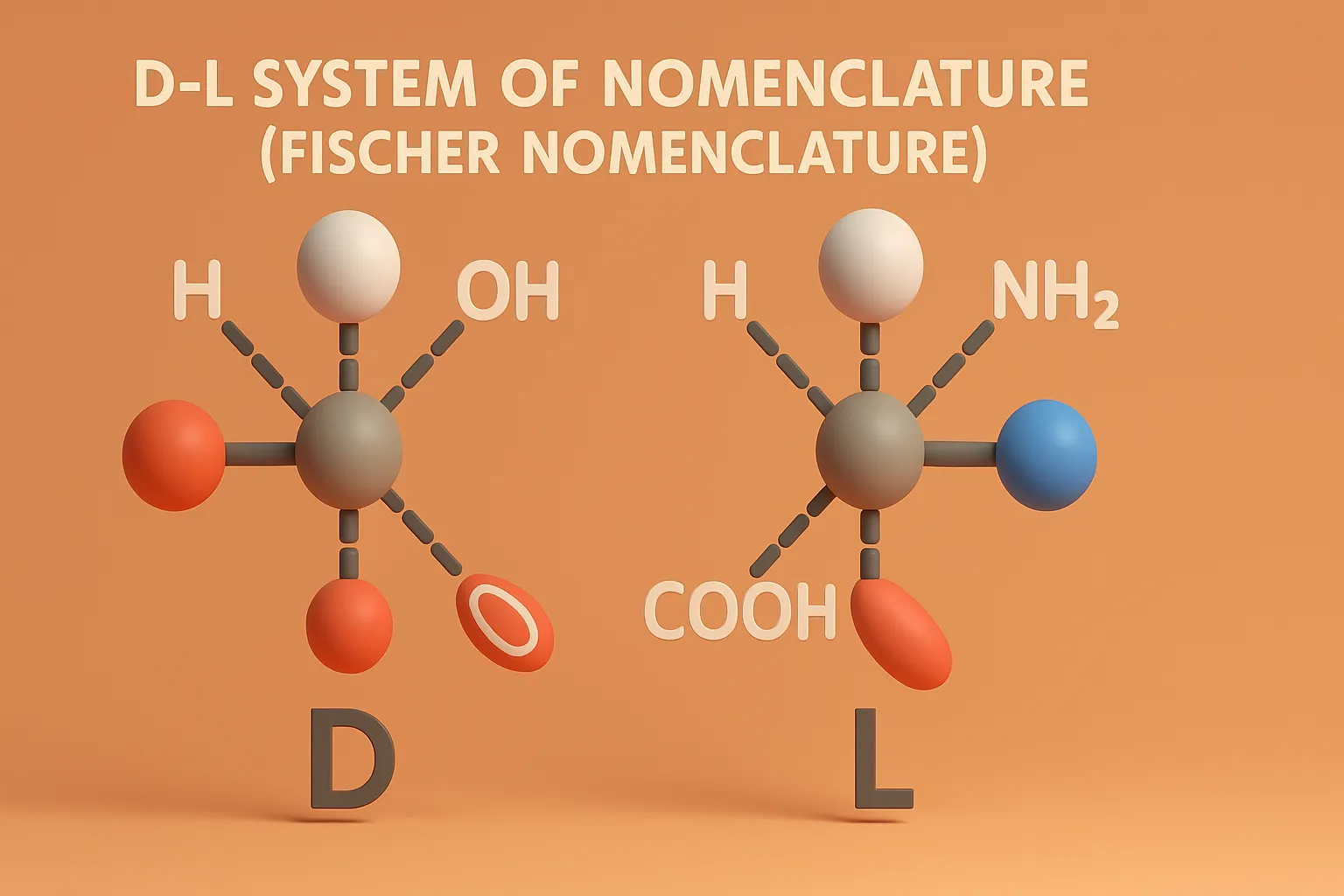
D-L System of Nomenclature (Fischer Nomenclature) classifies chiral molecules based on the spatial arrangement of groups around the asymmetric carbon.
What is it?
- The D-L system is a historical method used to designate the configuration of chiral molecules, particularly sugars and amino acids.
- It is based on the molecule’s similarity to glyceraldehyde, the simplest chiral molecule.
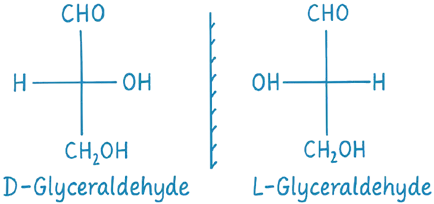
Reference Standard: Glyceraldehyde
- Glyceraldehyde has one chiral center and exists in two enantiomeric forms:
- D-Glyceraldehyde: The OH group on the chiral carbon is on the right in the Fischer projection.
- L-Glyceraldehyde: The OH group on the chiral carbon is on the left in the Fischer projection.
Fischer Projection Rules
- Vertical lines = bonds going into the plane (away from you)
- Horizontal lines = bonds coming out of the plane (toward you)
Advertisements
Assigning D or L:
- Draw the molecule in Fischer projection (vertical bonds go back, horizontal come out).
- Locate the chiral center farthest from the carbonyl group (for sugars) or the alpha-carbon (for amino acids).
- If the –OH (or –NH₂ in amino acids) is on the right → D-form, if on the left → L-form.
Important:
- D/L is not related to optical rotation.
- A D-compound can be (+) or (−); likewise for L.
Example:
- D-glucose: –OH on the right at the last chiral center
- L-glucose: –OH on the left at the last chiral center
Click Here to Watch the Best Pharma Videos
Advertisements